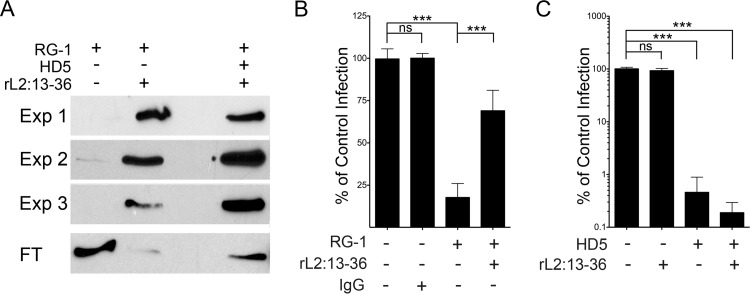
FIG 3.
HD5 does not block RG-1-L2 epitope binding. (A) RG-1 was immunoprecipitated by rL2:13–36 in the presence or absence of 5 μM HD5. Shown are bound antibody from three independent experiments (Exp 1 to 3) and a representative unbound fraction (FT) from one experiment visualized by immunoblotting. (B) Excess rL2:13–36 rescues HPV16 from RG-1 neutralization. Infection of HeLa cells by HPV16 PsV incubated with RG-1 alone or in competition with a 500-fold molar excess of rL2:13–36 was quantified relative to infection in the absence of inhibitor. BSA was used to normalize protein levels in all samples, and mouse IgG1 was used as an isotype control for RG-1. Data are means ± SD from three independent experiments. ***, P < 0.0001. (C) rL2:13–36 does not rescue HPV16 infection from HD5 neutralization. Infection of HeLa cells by HPV16 PsV incubated with 5 μM HD5 alone or in competition with a 500-fold molar excess of rL2:13–36 was quantified relative to infection in the absence of inhibitor. BSA was used to normalize protein levels in all samples. Data are means ± SD from three independent experiments. ***, P < 0.0001.