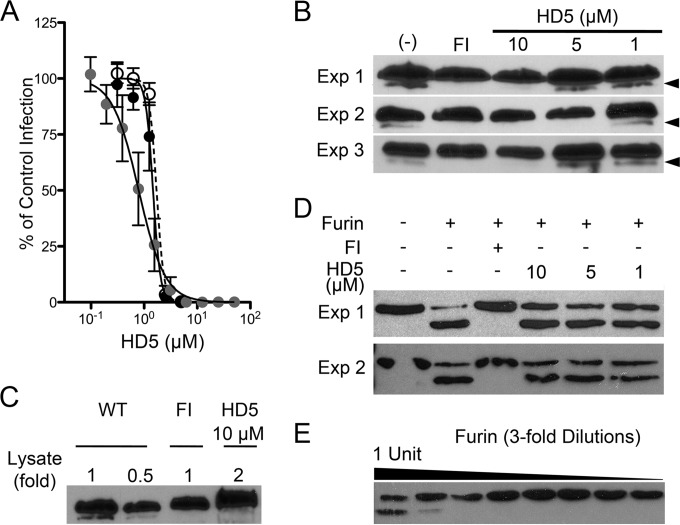
FIG 5.
(A) Myc-L2-HA HPV16 PsV is sensitive to HD5. HeLa cells were infected with WT HPV16 PsV in SFM (black circles, IC50 = 1.47 μM, 95% CI = 1.35 to 1.6 μM), Myc-L2-HA PsV in SFM (open circles, IC50 = 1.71 μM, 95% CI = 1.6 to 1.8 μM), or 10 times as much Myc-L2-HA PsV in complete medium (gray circles, IC50 = 0.79 μM, 95% CI = 0.69 to 0.9 μM) incubated with increasing concentrations of HD5. Data are means ± SD from three independent experiments normalized to infection in the absence of inhibitor. (B) HD5 inhibits furin cleavage of L2. HeLa cells were infected with Myc-16L2-HA HPV16 PsV in the presence of 40 μM furin inhibitor (FI), 1 to 10 μM HD5, or no inhibitor (−). L2 cleavage was assessed by immunoblotting of cell lysates 16 h p.i. using an anti-HA antibody. Cleaved L2 (arrow) is visible as a faster-migrating band below uncleaved L2. Shown are three independent experiments. (C) Analysis of a greater amount of lysate confirms the inhibition of furin cleavage. Different amounts of HD5-treated HPV16 PsV lysate, indicated by fold change relative to the amounts loaded in panel B, were assessed by immunoblotting with anti-HA antibody. (D) HD5 does not directly affect the enzymatic activity of furin. A total of 1.8 ng rL2:1–160 was digested with 1 U of furin in the presence or absence of the indicated inhibitors for 1 h at 30°C. Samples were immunoblotted using an anti-His antibody. Cleaved rL2:1–160 is the faster-migrating band. Shown are two independent experiments. (E) Titration of furin required for rL2:1–160 cleavage. rL2:1–160 was digested with a 3-fold dilution series of furin, starting at 1 U of total furin. Samples were resolved on a reducing gel and immunoblotted using anti-His antibody. Cleaved rL2:1–160 is the faster-migrating band.