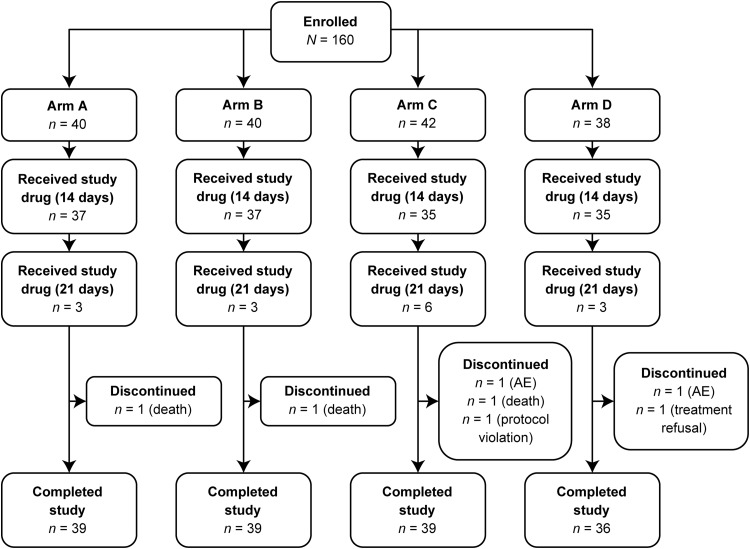
FIG 2.
Study design. Arm A, oral isavuconazole at 200 mg on day 1; once-daily oral isavuconazole at 50 mg thereafter. Arm B, oral isavuconazole at 400 mg on days 1, 7, and 14 and then day 21, if required. Arm C, oral isavuconazole at 400 mg on day 1 and once-daily oral isavuconazole at 100 mg thereafter. Arm D, oral fluconazole at 200 mg on day 1 and then once-daily oral fluconazole at 100 mg thereafter. AE, adverse event.