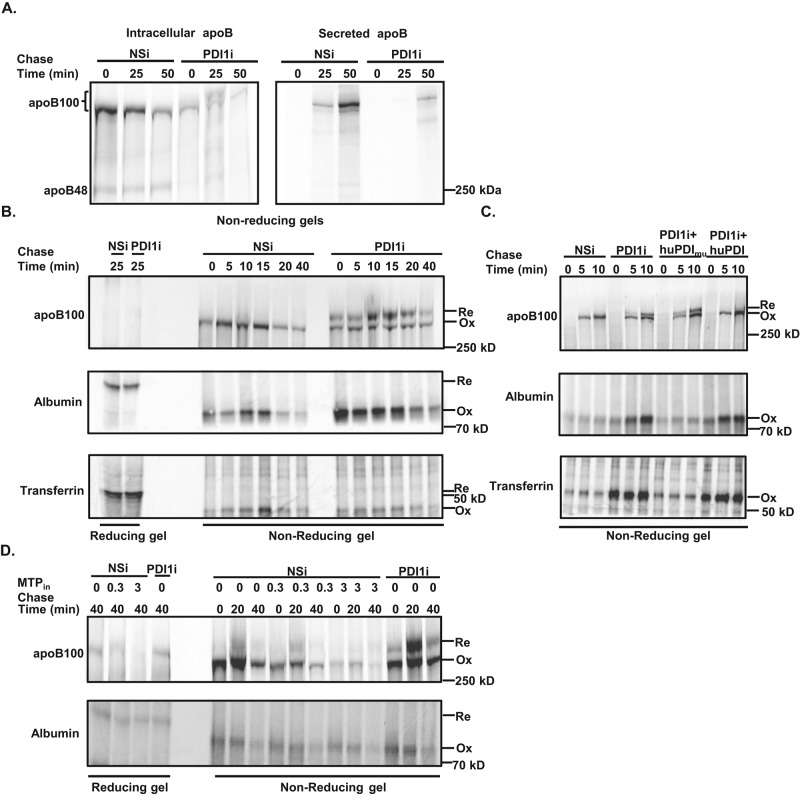
FIGURE 4:
Knockdown of Pdi1 decreases apoB100 oxidative folding in McA cells. (A) Pdi1 knockdown decreases oxidative folding of apoB100. Pulse-chase analysis of intracellular and secreted apoB100 in control (NSi) and Pdi1-knockdown (PDI1i) McA cells by nonreducing gel electrophoresis. The bracket indicates both oxidized and reduced form of apoB100 in nonreducing gel. (B) Pdi1-knockdown selectively reduces oxidative folding of apoB100. Oxidative folding of intracellular apoB100, albumin, and transferrin were measured by reducing (first two lanes) and nonreducing gel electrophoresis. Ox, oxidized form, and Re, reduced form, of the protein. (C) Oxidative folding of apoB100 requires catalytically active PDI1. Oxidative folding of intracellular apoB100, albumin, and transferrin was measured by nonreducing gel electrophoresis in control (NSi) cells, Pdi1-knockdown (PDI1i) and Pdi1-knockdown (PDI1i) cells with exogenous expression of HA-tagged substrate-trap mutant (huPDImu) or wild-type (huPDI) human PDI1. (D) Oxidative folding of apoB100 does not require MTP activity. Oxidative folding of intracellular apoB100 and albumin were analyzed by nonreducing gel electrophoresis in the absence or presence of an MTP inhibitor (CP-10447) at 0, 0.3, or 3 μM.