FIGURE 5:
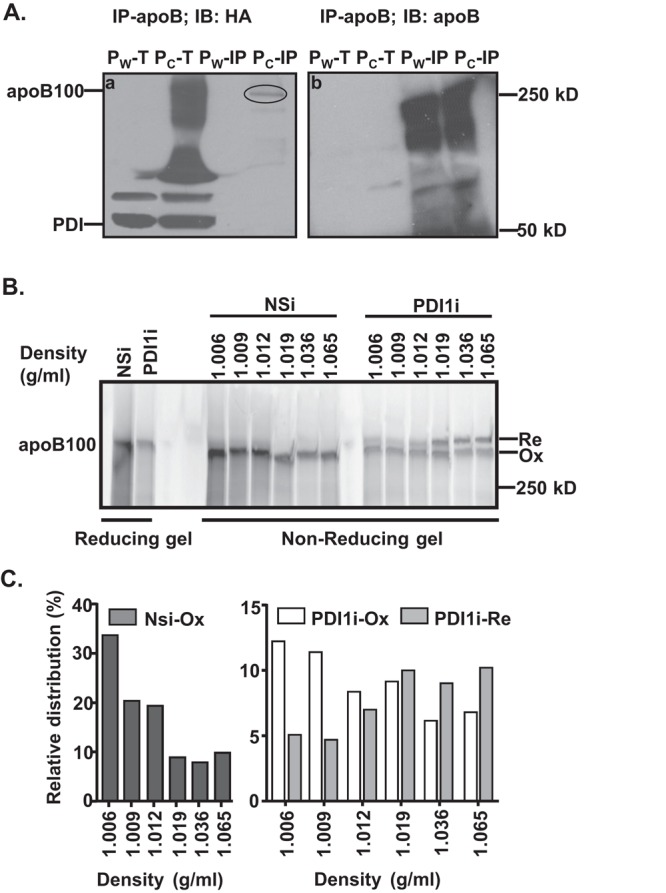
Reduced apoB100 oxidative folding decreases apoB lipidation. (A) PDI1 forms intermolecular disulfide bond(s) with apoB100. Lysates from cells that stably express HA-tagged wild-type (Pw: HA-PDI1) or substrate-trap mutant (Pc: HA-PDICXXA) of human PDI1 were immunoprecipitated with apoB100 antibody and analyzed by nonreducing SDS–PAGE and immunoblotting against HA antibody. The circle indicates PDI1 complexed with apoB100. IP, immunoprecipitated protein complex; T, total cell lysates. (B) Impaired apoB100 oxidative folding decreases apoB100 lipidation. Secreted apoB100-containing lipoproteins from McA cells were separated by DGUC as described in Figure 3 and analyzed by nonreducing gel electrophoresis. (C) The amount of apoB100 in each fraction was quantified by ImageJ. Relative distributions were calculated according to the percentage of apoB100 in each fraction relative to its total intensity across the gel.
