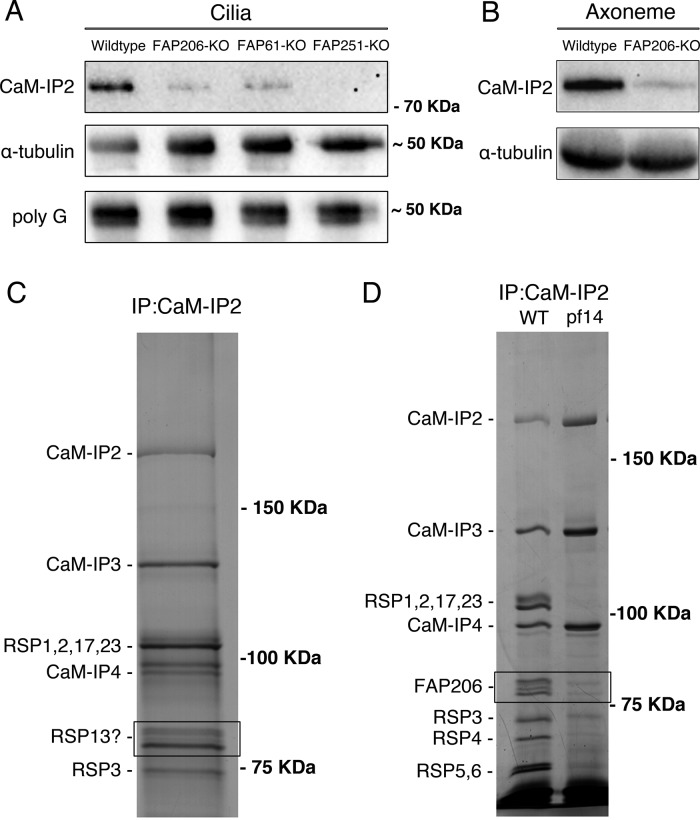
FIGURE 7:
FAP206 interacts with CSC. (A) A Western blot of purified cilia isolated from Tetrahymena strains that are wild type, lack genes encoding the Tetrahymena homologues of CSC proteins (FAP61/CaM-IP3 or FAP251/CaM-IP4), or lack FAP206, probed with antibodies specific to FAP91/CaM-IP2 of C. reinhardtii (top; Dymek et al., 2011), 12G10 monoclonal α-tubulin (middle), or polyglycylated tubulin (bottom). Note that the levels of the anti-FAP91/CaM-IP2 band are strongly reduced in the strains lacking the CSC protein homologues, indicating that the antibodies are specific to the Tetrahymena FAP91 homologue. Furthermore, the levels of the FAP91 homologue are strongly reduced in the strain lacking FAP206, indicating that axonemal assembly of FAP91 and likely other CSC components depends on the presence of FAP206. Images of the entire blots are shown in Supplemental Figure S1. (B) Western blot of axonemes of wild type and FAP206-KO probed with anti-FAP91 and 12G10 anti–α-tubulin antibodies. (C) Silver stained SDS–PAGE gels showing an immunoprecipitate obtained from the radial spoke-enriched supernatants of C. reinhardtii axonemes. The positions of known CSC components and major radial spoke proteins are marked. The two bands (boxed area) that migrated with an apparent molecular weight of 80 kDa were found to contain the Chlamydomonas FAP206 homologue (CHLREDRAFT_171124). (D) Immunoprecipitates obtained with the anti-FAP91/CaM-IP2 antibodies from the radial spoke–enriched supernatant of Chlamydomonas axonemes that were either wild type or spokeless pf14 mutant. Note the absence of the FAP206 bands, indicating that the CSC-FAP206 interaction requires RSP3 as an intermediate.