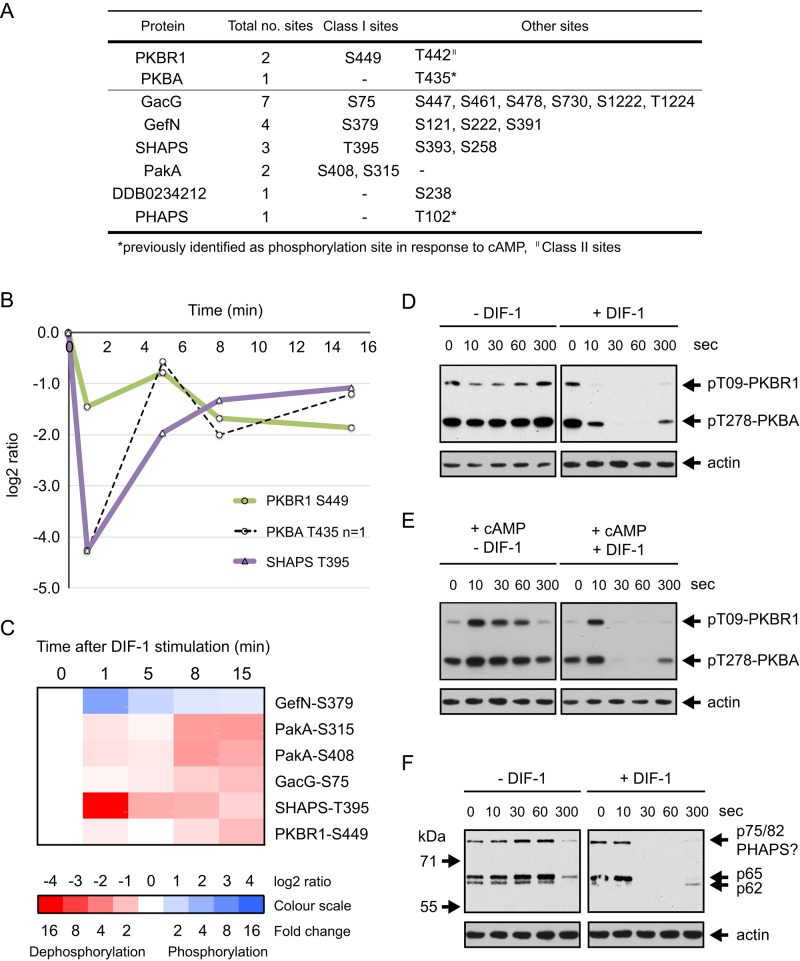
FIGURE 6:
DIF-1 induced dephosphorylation of PKB homologues and their substrates. (A) Summary of DIF-1–regulated phosphorylation sites in PKBA, PKBR1, and their substrates. (B) Temporal profile for DIF-1–induced phosphorylation changes in class I sites on PKBA, PKBR1, and their substrate SHAPS (DDB0306661). Solid lines represent averaged data for class I sites and dashed lines are from class III sites from a single experiment. (C) Heat-map representation of the temporal phosphorylation changes of class I DIF-1–regulated phosphorylation sites in PKB substrates. Averaged values, n ≥ 2. (D) PKB AL phosphorylation in response to DIF-1. Ax2 cells starved for 5 h were treated ± 100 nM DIF-1. Results are representative of at least three independent experiments. (E) Antagonism between cAMP and DIF-1. Cells were starved as in D and then treated with 1 μM cAMP ± 100 nM DIF-1. Samples collected at the times indicated and then immunoblotted as in D. (F) The response of PKB substrate phosphorylation to DIF-1. Samples from D were immunoblotted with anti-phosphospecific PKB substrate antibody. Blots were stripped and reprobed with anti-actin antibody as a loading control.