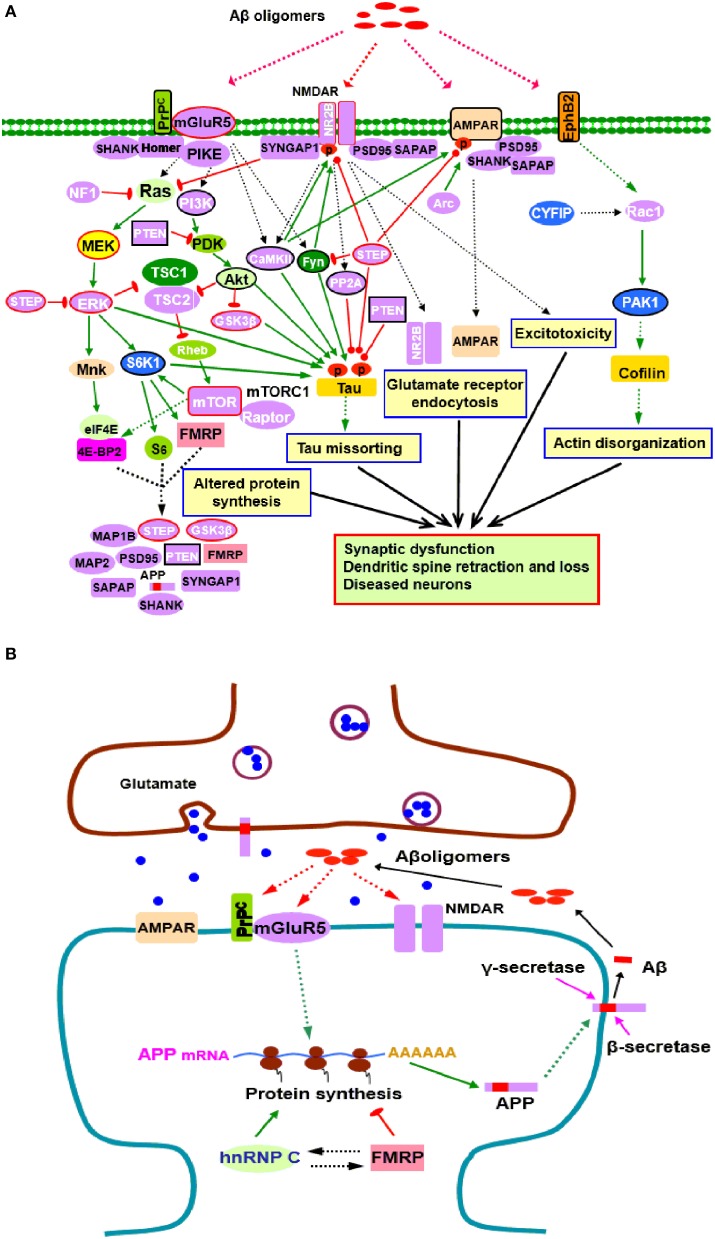
Figure 1.
Potential roles of FMRP in the pathogenesis of AD. (A) FMRP might be involved in oligomeric Aβ induced neurotoxicity. At pathological concentrations, Aβ oligomers may interact with multiple neuronal synaptic receptors such as mGluR5-PrPC, NMDARs, AMPARs, and EphB2, triggering a series of toxic synaptic events which may involve FMRP and eventually lead to synaptic dysfunction and neuronal loss. These events include: Aberrant activation of PI3K-Akt-mTORC1 and MEK-ERK signaling pathways linked to cap-dependent translation result in altered synthesis of synaptic proteins; Oligomeric Aβ exposure disrupts the balance between tau kinase (GSK3β, CaMKII, Akt, Fyn, and ERK1/2) and phosphatase (PP2A, STEP, and PTEN) activities, inducing tau hyperphosphorylation and aggregation; Stimulating EphB2-Rac1/PAK1 signaling by Aβ oligomers induces cofilin phosphorylation and actin depolymerization, leading to actin network disorganization; Binding of Aβ oligomers to PrPC-mGluR5 activates Fyn kinase which phosphorylates not only tau, but also NR2B subunit of NMDARs, enhancing NMDAR activity and causing excitotoxicity; STEP is also activated, inactivates Fyn, and dephosphorylates AMPARs and NMDARs, resulting in endocytosis of glutamate receptors, a cellular process involves Arc, PSD-95, SAPAP, and other synaptic proteins. Purple proteins are those whose mRNAs are FMRP targets (Darnell and Klann, 2013; Pasciuto and Bagni, 2014b; Santini and Klann, 2014); the blue ones are the interacting proteins of FMRP (Pasciuto and Bagni, 2014a). Proteins with red lines around them indicate those that have been successfully manipulated either pharmacologically or genetically to reverse molecular, cellular and/or behavioral phenotypes in animal models of AD (Zhang et al., 2010; Malinow, 2012; Caccamo et al., 2014; Feld et al., 2014; Hamilton et al., 2014; Llorens-Martin et al., 2014) as well as ASDs (Goebel-Goody et al., 2012; Guo et al., 2012; Won et al., 2012; Darnell and Klann, 2013; Osterweil et al., 2013; Wang and Doering, 2013; Wang, 2014). Proteins with black lines around them are the ones that have been reported to be potential targets for AD therapy (Griffin et al., 2005; Lafay-Chebassier et al., 2005; Ma et al., 2008; Cisse et al., 2011; Moriguchi, 2011; Chang et al., 2012; Gross and Bassell, 2014; Nygaard et al., 2014; Sontag and Sontag, 2014). (B) FMRP in APP synthesis. Aβ oligomers stimulate dendritic APP synthesis through PrPC-mGluR5 mediated protein translation dependent pathway, providing template for secretase cleavage to produce Aβ and other metabolites. A positive feedback may exist whereby production of APP results in increased substrate for amyloidogenic processing and release of Aβ, which then acitivates mGluR5 to further stimulate APP translation. In this process, FMRP competes with the other RNA binding protein hnRNP C to modulate APP translation. FMRP is a repressor of APP translation, whereas hnRNP C acts as an enhancer. The rate of APP synthesis is directly influenced by the relative association of each RNA binding protein (Lee et al., 2010). In signaling pathways, arrows indicate positive (green) or inhibitory (red) consequence on downstream components, but they do not necessarily represent direct interactions.