Figure 5. Photocatalytic hydrogen production.
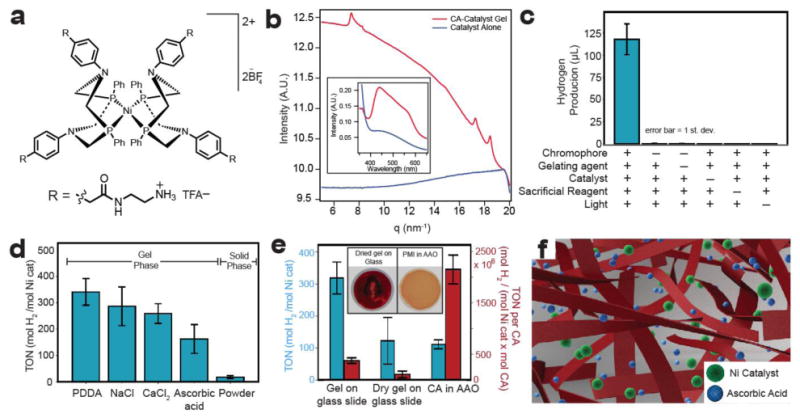
a, Chemical structure of water-soluble proton-reduction catalyst. b, WAXS experiments demonstrate that the addition of catalyst to CA induces crystallization and induces stronger electronic coupling between CA molecules (observed by absorbance spectroscopy, inset). c, H2 evolution experiments show that CA-PDDA gel is capable of photosensitizing nickel catalyst for H2 evolution. No H2 is evolved when chromophore, catalyst, sacrificial reagent, or light is omitted from the system. d, H2 production histogram of CA gels prepared with NaCl, PDDA, CaCl2, and ascorbic acid compared to insoluble, protonated CA. All gel phases produce more H2 than the solid phase CA powder. e, H2 production from CA-PDDA gels prepared on a glass slide, dried on a glass slide (inset, left), and from CA in an AAO (anodized aluminum oxide) filter (inset, right). In one case the data are normalized per mole catalyst (TON) (left) and per mole catalyst and mole chromophore (TON per CA) (right). f, Schematic of gel showing that CA nanoribbons (in red) trap solvent water molecules (not shown for clarity) within a three-dimensional architecture. This open architecture allows integration of ascorbic acid and catalyst with CA ribbons for photoinduced H2 production.
