Abstract
AIM: To evaluate the clinical usefulness of soluble heparin-binding epidermal growth factor (sHB-EGF) as a serum biomarker for gastric cancer (GC).
METHODS: Serum sHB-EGF levels were measured by a commercially available human HB-EGF ELISA Kit and compared among 60 normal controls, 30 high-risk patients, 37 early gastric cancer (EGC), and 30 advanced gastric cancer (AGC) through ANOVA test. Correlations between serum sHB-EGF and clinicopathological features of GC were analyzed through Spearman’s correlation. The diagnostic performance of serum sHB-EGF for GC was evaluated through receiver operating characteristic (ROC) curve and logistic regression analysis.
RESULTS: Serum sHB-EGF levels were significantly higher in AGC group (314.4 ± 127.5 pg/mL) than EGC (165.3 ± 123.2 pg/mL), high-risk (98.7 ± 67.3 pg/mL), and control (94.7 ± 83.6 pg/mL) groups (post-hoc Bonferroni, all P < 0.001), respectively. Serum sHB-EGF levels were also significantly higher in EGC group than high-risk (P = 0.049) and control (P = 0.006) groups. Clinicopathologically, serum sHB-EGF levels closely correlated with depth of invasion (T-stage, γs = 0.669, P < 0.001), lymph node metastasis (N-stage, γs = 0.407, P = 0.001), and distant metastasis (M-stage, γs = 0.261, P = 0.030). ROC curve and logistic regression analysis demonstrated a remarkable diagnostic potential of serum sHB-EGF.
CONCLUSION: Serum sHB-EGF is closely correlated with advanced stage GC and can be a promising serological biomarker for GC.
Keywords: Biomarker, Diagnostic, Gastric cancer, Prognostic, Soluble heparin-binding EGF-like growth factor
Core tip: Early detection of gastric cancer (GC) is most important issue. Although endoscopic examination is an ideal, highly reliable technique for early detection of GC, it has limitation because of its high cost and invasiveness. Therefore, inexpensive, comfortable, reliable and less-invasive biomarkers need to be identified. Here, we reported that serum levels of soluble HB-EGF (sHB-EGF) closely correlated with advanced TNM stage and was higher in EGC than high-risk group. We also identified a remarkable diagnostic accuracy of serum sHB-EGF for GC. To our knowledge, this is the first study to validate sHB-EGF as a desirable serum biomarker for GC.
INTRODUCTION
Although the incidence of gastric cancer (GC) has decreased over the past few decades, it is still a serious health problem because it is the second most frequent cause of cancer-related deaths worldwide[1], which may be originated from that the prognosis of advanced gastric cancer (AGC) remains poor despite the recent advances in treatment strategies[2]. In contrast, the prognosis of early gastric cancer (EGC) is favorable[3]. These facts strongly support the clinical importance of early detection of GC. Endoscopic examination is an ideal, highly reliable technique for early detection of GC and its premalignant lesions[4]. However, its usefulness as a routine screening method is somewhat limited because of its high cost and the risk associated with this invasive procedure. Therefore, inexpensive, comfortable, reliable and less-invasive biomarkers such as accurate serological biomarker need to be identified.
Carcinoembryonic antigen (CEA), a well-known gastrointestinal tumor-related biomarker, was initially applied as a biomarker for GC. However, recent studies have found that CEA does not demonstrate the sensitivity or specificity needed to effectively screen for GC[5].
Activation of the epidermal growth factor (EGF) and epidermal growth factor receptor (EGFR) families is known to be associated with the progression of various tumor types[6]. Activation of EGF-EGFR axis is also associated with tumor growth, serosal invasion, and resultant poor prognosis of GC patients[7-9]. EGFR has seven ligands. Of these ligands, heparin-binding EGF-like growth factor (HB-EGF) is in particular thought to be associated with GC development and progression[10-13].
HB-EGF is a member of the EGF family[14]. It is initially synthesized as a membrane-anchored form (pro-HB-EGF), which is subsequently cleaved from the membrane by metalloproteinase to produce a mature soluble form of HB-EGF (sHB-EGF)[15]. Many in vitro studies demonstrated that sHB-EGF is a potent mitogen for several types of epithelial cells[16-19]. Several studies also demonstrated that HB-EGF is overexpressed in human GC cell lines and GC tissues[13,19,20]. Therefore, this growth factor has potentials as a biomarker for GC. Although tissue markers have high specificity, reproducibility, and reliability, serological biomarkers are preferable as a screening method for GC because tissue markers require invasive techniques such as endoscopy and biopsy. Because HB-EGF is released into circulation as a mature soluble form, this growth factor can be measured in serum, and serum levels of this soluble factor may reflect the disease progression in GC. However, there is little information about the serological levels of sHB-EGF according to gastric carcinogenic sequence.
In this study, we determined how serum levels of sHB-EGF related to the “gastritis-dysplasia-carcinoma” sequence of gastric carcinogenesis[21] and analyzed its correlations with clinicopathological features of GC. We also investigated the usefulness as a biomarker for GC compared with serum CEA.
MATERIALS AND METHODS
Subjects and clinical information
A total of 157 subjects from Yonsei University Health System were enrolled in this study. All subjects underwent upper gastrointestinal endoscopy (Types XQ-260, Olympus, Tokyo, Japan) with biopsy. The final diagnosis was made based on histological findings from biopsy or surgical specimens. All patients were diagnosed for the first time during the enrollment period, and blood samples were collected before they received any treatments. Blood samples were stored as serum fractions at -80 °C until analysis. The Institutional Review Board of Yonsei University Health System approved the current study, and written informed consent was obtained from all participants in accordance with the Declaration of Helsinki.
Subjects who suffered from chronic diseases such as liver cirrhosis, chronic renal disease, and diabetes mellitus were excluded from this study. Subjects with other cancers and other gastric neoplasms such as gastrointestinal stromal tumors, mucosa-associated lymphoid tissue lymphomas, and neuroendocrine tumors were also excluded. Patients who previously received any treatment for GC or its premalignant lesions were also excluded.
Subjects were classified into the following four groups according to the “gastritis-dysplasia-carcinoma” sequence of gastric carcinogenesis[21]: control group, which included normal mucosa or acute and chronic gastritis; high-risk group, which included intestinal metaplasia (IM) and dysplasia; EGC group; and AGC group. Both age and sex were matched in all groups. All patients in the cancer groups underwent imagining studies including chest X-ray, abdominal-pelvic helical computed tomography, and whole-body positron emission tomography to determine TNM stage. TNM stage for GC was evaluated according to the 7th International Union Against Cancer-TNM stage guidelines for GC[22] based on radiological studies or surgical findings. Helicobacter pylori (H. pylori) infection was determined by staining of gastric tissue with Giemsa solution (Sigma, MO, United States). Glandular atrophy and IM were diagnosed according to the updated Sydney classification[23], and pathological determination of differentiation status (well, moderate, poor, and signet-ring cell) was performed according to the Lauren classification.
Measurement of serum CEA and HB-EGF levels
Serum CEA levels were measured by the Beckman Access CEA assay (Beckman Coulter Inc., MN, United States). Serum sHB-EGF levels were measured by a commercially available human HB-EGF ELISA Kit (DY259, RD, MN, United States) according to the manufacturer’s instructions. Briefly, 96-well microplates were coated with capture antibody (80 μg/well, goat anti-human HB-EGF) at 4 °C for 16 h. After washing, the plates were blocked with Reagent Diluent (provided in kit) and then incubated for 1 h at room temperature (RT). After washing, 100 μL of diluted sample, standard, and control were added to each well. The microplates were then incubated for 2 h at RT. Subsequently, microplates were washed and then detection antibody was added (10 ng/well, biotinylated goat anti-human HB-EGF). The plates were then incubated for 2 h at RT. After washing, streptavidin-HRP was added and incubated for 20 min at RT in the dark place. Plates were then washed again, and 100 μL of chromogen (H2O2 and tetra-methylbenzidine) was added to each well. The enzyme reaction proceeded for 20 min at RT in the dark place. The chromogenic substrate reaction was stopped by the addition of stop solution (2 mol/L H2SO4) and the absorbance was read at 450/570 nm. The final values were calculated based on a calibration curve prepared from standards. The ELISA for sHB-EGF levels was tested in triplicate.
Statistical analysis
To calculate the appropriate sample size for each group, Russ Lenth’s interactive power/sample size online calculator was used. Under assuming that there were 4 comparison groups, the estimated standard deviation (SD) was 1, and the confidence level was 0.05, sample size of ≥ 30 in each group achieved a statistical power > 80% using one-way analysis of variance (ANOVA).
For statistical analysis for current data, SPSS version 20.0 (IBM Corp., NY, United States) was used. P values < 0.05 were considered statistically significant. Values (sHB-EGF, CEA) were expressed as the mean with the 25%-75% SD. Means of each group was compared by ANOVA test with multiple comparisons by using the post-hoc Bonferroni method. An independent sample t-test was used to compare the mean between the cancer groups vs non-cancer groups. Spearman’s correlation (coefficient, γs) was used to assess the relationship between continuous variables and non-continuous variables, and Pearson’s correlation (coefficient, γp) was used to assess the relationship between continuous variables. Nominal data were compared by χ2 test. The receiver operating characteristic (ROC) curves was conducted and area under the curve (AUC) was calculated to compare the diagnostic accuracy between serum sHB-EGF and serum CEA. Logistic regression analysis was performed to obtain the best sensitivity/specificity to predict the presence of GC as a single-marker or as a part of multiple-markers panel. Each marker was included as a linear term.
RESULTS
Baseline characteristics of subjects and serum levels of sHB-EGF and CEA according to disease groups
The 157 subjects are composed of 60 individuals/patients with normal mucosa or gastritis (control group), 30 patients with IM/dysplasia (high-risk group), 37 patients with EGC (EGC group), and 30 patients with AGC (AGC group). The control group was further subdivided into two subgroups; patients with normal mucosa/chronic superficial gastritis (CSG, n = 30) and patients with chronic atrophic gastritis (CAG, n = 30) because the risk of GC development was different between CSG and CAG. The normal mucosa/CSG group was also further subdivided into normal mucosa (n = 15) or CSG (n = 15) because gastric inflammation status may affect sHB-EGF levels comparing to normal mucosa. The clinical and histopathological features of subjects in each group are described in Table 1. There were no significant differences in distribution of age and sex, and the status of H. pylori infection among the disease groups (χ2, all P > 0.05). In the cancer groups, the location of primary tumor did not differ (P > 0.05), while histological differentiation, primary tumor size, and TNM stage were significantly different between the EGC and AGC groups (all P < 0.05).
Table 1.
Baseline characteristics of subjects in each group
| Groups | Control2 (n = 60) | High-risk3 (n = 30) | EGC (n = 37) | AGC (n = 30) | P value4 |
| Clinical features | |||||
| Age (mean ± SD, yr) | 56.5 ± 11.1 | 66.2 ± 7.6 | 58.3 ± 10.6 | 56.3 ± 10.3 | 0.856 |
| Sex (male:female, n) | 37:23 | 19:11 | 22:15 | 17:13 | 0.953 |
| H. pylori infection (-/+, n) | 35:25 | 17:13 | 22:15 | 20:10 | 0.857 |
| Histopathological features | |||||
| Histology (well:mod:poorly:signet) | NS | NS | 14:9:7:7 | 2:6:16:6 | 0.006 |
| Tumor location (lower:middle:upper)1 | NS | NS | 20:13:4 | 18:9:3 | 0.884 |
| Size of tumor (mean ± SD, cm) | NS | NS | 3.9 ± 1.2 | 5.0 ± 1.3 | 0.010 |
| T-stage (T1a:T1b:T2:T3:T4) | NS | NS | 31:6:0:0:0 | 0:0:15:4:11 | < 0.001 |
| N-stage (N0:N1:N2:N3) | NS | NS | 35:2:0:0 | 15:2:4:9 | < 0.001 |
| Distant metastasis (M0:M1) | NS | NS | 37:0 | 25:5 | < 0.001 |
| Overall stage (I:II:III:IV) | NS | NS | 37:0:0:0 | 7:12:6:5 | < 0.001 |
Tumor location is divided into three areas: lower third (antrum-angle), middle third (low body-middle body), and upper third (upper body-cardia).
Control group includes individuals with normal mucosa or patients with simple chronic superficial gastritis and chronic atrophic gastritis.
High-risk group included patients with intestinal metaplasia and dysplasia.
Continuous data were compared by ANOVA test and nominal data by χ2 test. P < 0.05 (two-tailed) was considered statistically significant. AGC: Advanced gastric cancer; EGC: Early gastric cancer; H. pylori: Helicobacter pylori; Mod: Moderate-differentiated carcinoma; Poorly: Poorly-differentiated carcinoma; SD: Standard deviation; Signet: Signet ring cell carcinoma; Well: Well-differentiated carcinoma.
Serum sHB-EGF levels increased along the GC carcinogenic sequence, and the differences among the groups were statistically significant (ANOVA, P < 0.001; Table 2). Serum sHB-EGF levels were significantly higher in the AGC group (314.4 ± 127.5 pg/mL) compared with those of EGC (165.3 ± 123.2 pg/mL), high-risk (98.7 ± 67.3 pg/mL), and control (94.7 ± 83.6 pg/mL) groups (post-hoc Bonferroni, all P < 0.001), respectively. Serum sHB-EGF levels were also significantly higher in the EGC groups than the high-risk (P = 0.049) and control (P = 0.006) groups, respectively. However, there was not a significant difference between the high-risk and control groups (P > 0.05). Serum sHB-EGF levels were also not significantly different between the CAG and CSG groups (P > 0.05), or between the CSG and normal mucosa groups (P > 0.05), respectively (Table 3). On the other hand, serum CEA levels were not significantly different among the control, high-risk, and EGC groups. The AGC group was the only population with significantly elevated serum CEA levels (P = 0.001), especially in the case with distant metastasis. When serum sHB-EGF levels were compared between cancer and non-cancer groups, they were significantly higher in the cancer groups (232.1 ± 144.9 pg/mL) than in the non-cancer groups (96.0 ± 78.2 pg/mL; t-test, P < 0.001; Table 4).
Table 2.
Serum levels of soluble heparin-binding epidermal growth factor-like growth factor and carcinoembryonic antigen according to disease groups
| Groups | Control2 (n = 60) | High-risk3 (n = 30) | EGC (n = 37) | AGC (n = 30) | P value4 |
| Serum sHB-EGF1 (pg/mL) | 94.7 ± 83.6 | 98.7 ± 67.3 | 165.3 ± 123.2 | 314.4 ± 127.5 | < 0.001 |
| Serum CEA1 (ng/mL) | 1.8 ± 1.5 | 2.2 ± 1.1 | 2.4 ± 1.4 | 4.5 ± 5.1 | 0.001 |
All tested values are expressed as the mean ± standard deviation.
Control group includes individuals with normal mucosa or patients with simple chronic superficial gastritis and chronic atrophic gastritis.
High-risk group included patients with intestinal metaplasia and dysplasia.
One-way analysis of variance (ANOVA) test with the multiple comparisons by the post-hoc Bonferroni method is applied to compare the differences in means among disease groups. P < 0.05 (two-tailed) was considered statistically significant. AGC: Advanced gastric cancer; EGC: Early gastric cancer; CEA: Carcinoembryonic antigen; sHB-EGF: Soluble heparin-binding EGF-like growth factor.
Table 3.
Serum levels of soluble heparin-binding epidermal growth factor-like growth factor between normal mucosa/chronic superficial gastritis and chronic atrophic gastritis or between normal mucosa and chronic superficial gastritis
| Groups | Serum sHB-EGF1 (pg/mL) |
| Normal mucosa/CSG (n = 30) | 86.4 ± 73.5 |
| CAG (n = 30) | 102.9 ± 93.1 |
| P value2 | 0.449 |
| Normal mucosa (n = 15) | 83.1 ± 59.0 |
| CSG (n = 15) | 89.7 ± 87.6 |
| P value2 | 0.811 |
Tested value is expressed as the mean ± SD.
An independent sample t-test is applied to compare the differences of means between two groups. P < 0.05 (two-tailed) was considered statistically significant. sHB-EGF: Soluble heparin-binding EGF-like growth factor. CSG: Chronic superficial gastritis; CAG: Chronic atrophic gastritis.
Table 4.
Serum levels of soluble heparin-binding epidermal growth factor-like growth factor and carcinoembryonic antigen between non-cancer and cancer groups
| Groups | Non-cancer2 (n = 90) | Cancer3 (n = 67) | P value4 |
| Serum sHB-EGF1 (pg/mL) | 96.0 ± 78.2 | 232.1 ± 144.9 | < 0.001 |
| Serum CEA1 (ng/mL) | 1.9 ± 1.4 | 3.3 ± 3.7 | 0.004 |
All tested values are expressed as the mean ± SD.
Non-cancer groups include normal/gastritis group and IM/dysplasia group.
Cancer groups include early gastric cancer (EGC) and advanced gastric cancer (AGC) groups.
An independent sample t-test is applied to compare the differences of means between non-cancer and cancer groups. P < 0.05 (two-tailed) was considered statistically significant. CEA: Carcinoembryonic antigen; sHB-EGF: Soluble heparin-binding EGF-like growth factor.
Correlations between serum sHB-EGF levels and clinicopathological characteristics of subjects
Table 5 shows that serum sHB-EGF levels were not affected by sex (γs = 0.138, P = 0.076) or the status of H. pylori infection (γs = -0.054, P = 0.486), whereas these levels were negatively correlated with age (γp = -0.265, P = 0.001). However, serum sHB-EGF levels were not closely correlated with age when analysis was performed in just non-cancer groups (γp = 0.108, P = 0.313; Table 6). In contrast, when analysis was performed within the cancer groups, the serum sHB-EGF levels were negatively correlated with age (γp = -0.314, P = 0.010). This result implies that serum sHB-EGF levels are affected by the age of patients with GC; relatively younger patients with GC had more highly elevated sHB-EGF levels compared with relatively older patients with GC. On the other hand, serum CEA levels were not affected by sex, age and the status of H. pylori infection (all P > 0.05; Table 5).
Table 5.
Correlations between serum soluble heparin-binding epidermal growth factor-like growth factor and clinicopathological characteristics of each group
| Clinicopathological characteristics | Serum sHB-EGF | Serum CEA |
| γs (P value) | γs (P value) | |
| Age (yr)1 | -0.265 (0.001) | -0.087 (0.275) |
| Sex (male:female) | 0.138 (0.076) | -0.021 (0.792) |
| H. pylori infection (-/+) | -0.054 (0.486) | -0.114 (0.151) |
| Histology (well:mod:poorly:signet) | 0.214 (0.078) | 0.090 (0.465) |
| Tumor location (lower:middle:upper) | -0.052 (0.652) | -0.048 (0.691) |
| T-stage (T1a:T1b:T2:T3:T4) | 0.669 (< 0.001) | 0.201 (0.101) |
| N-stage (N0:N1:N2:N3) | 0.407 (0.001) | 0.073 (0.552) |
| Distant metastasis (M0:M1) | 0.261 (0.030) | 0.328 (0.006) |
| Overall stage (I:II:III:IV) | 0.570 (< 0.001) | 0.229 (0.060) |
| Size of tumor (< 3 cm; 3-5 cm and > 5 cm)2 | 0.237 (0.048) | 0.362 (0.002) |
This value is continuous variable. Thus, correlation is evaluated by Pearson’s correlation (γp).
Subjects were classified into three groups according to primary GC size: < 3 cm, 3-5 cm, and > 5 cm to analyze the relationship between serum HB-EGF levels and primary GC size. CEA: Carcinoembryonic antigen; H. pylori: Helicobacter pylori; Mod: Moderate-differentiated carcinoma; Poorly: Poorly-differentiated carcinoma; Signet: Signet ring cell carcinoma; sHB-EGF: Soluble heparin-binding EGF-like growth factor; Well: Well-differentiated carcinoma; γs: Spearman’s correlation coefficient. P < 0.05 (two-tailed) was considered statistically significant. Statistically significant values are given in bold print.
Table 6.
Pearson’s correlation between serum soluble heparin-binding epidermal growth factor-like growth factor and age in non-cancer groups vs cancer groups
| Groups | Serum sHB-EGF | Serum CEA | |
| γp (P value) | γp (P value) | ||
| Non-cancer groups1 | Age | -0.108 (0.313) | 0.122 (0.265) |
| Cancer groups2 | Age | -0.314 (0.010) | -0.187 (0.133) |
Non-cancer groups include normal/gastritis and IM/dysplasia groups.
Cancer groups include EGC and AGC groups. γp: Pearson’s correlation coefficient. Statistically significant values are given in bold print. AGC: Advanced gastric cancer; EGC: Early gastric cancer; sHB-EGF: Soluble heparin-binding epidermal growth factor-like growth factor; CEA: Carcinoembryonic antigen.
Histopathologically, there were no significant relationships between serum sHB-EGF levels and the histological differentiation of GC (Lauren classification system), although sHB-EGF levels tend to be higher in diffuse-type than in intestinal-type (γs = 0.214, P = 0.078; Table 5). Serum sHB-EGF levels were not also affected by primary tumor location (γs = −0.054, P = 0.652; Table 5).
On the other hand, serum sHB-EGF levels were closely correlated with depth of invasion (T-stage, γs = 0.669, P < 0.001), lymph node metastasis (N-stage, γs = 0.407, P= 0.001), distant metastasis (M-stage, γs = 0.261, P = 0.030), and overall stage (γs = 0.570, P < 0.001) respectively (Table 5). To analyze the relationship between serum sHB-EGF levels and primary GC size, patients were divided into 3 groups based on the tumor size: < 3 cm, 3-5 cm, and > 5 cm. Table 5 shows that primary GC size was positively correlated with serum sHB-EGF levels (γs = 0.237, P = 0.048). On the other hand, serum CEA levels were only correlated with tumor size (γs = 0.382, P = 0.006) and distant metastasis (γs = 0.362, P = 0.002). Collectively, histopathological results suggest that serum sHB-EGF levels were closely correlated with advanced stage and poor prognosis of GC.
Diagnostic accuracy of serum sHB-EGF levels for prediction of GC
ROC curve was generated and AUCs were calculated to compare the diagnostic accuracy of serum sHB-EGF with serum CEA for prediction of GC (Figure 1). The AUC of serum sHB-EGF was 0.85 (95%CI: 0.79-0.91), and those of serum CEA was 0.64 (95%CI: 0.55-0.73). This analysis indicates that serum sHB-EGF has a higher diagnostic accuracy to predict the presence of GC compared with CEA.
Figure 1.
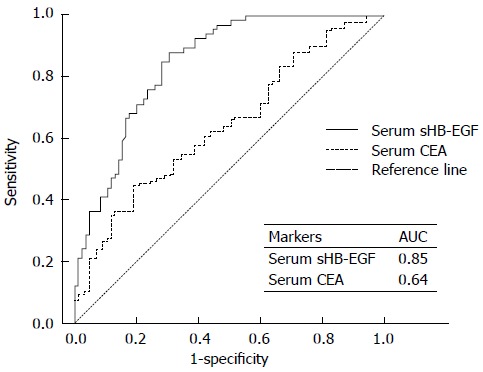
Receiver operating characteristic curve for serum soluble heparin-binding epidermal growth factor-like growth factor compared with the curve for serum carcinoembryonic antigen to predict gastric cancer. ROC: Receiver operating curve; sHB-EGF: Soluble heparin-binding epidermal growth factor-like growth factor; CEA: Carcinoembryonic antigen; AUC: Area under the ROC curve.
Logistic regression analysis further confirmed the remarkable diagnostic accuracy of serum sHB-EGF for GC; the sensitivity and specificity of serum sHB-EGF levels for diagnosis of GC were 76.1% and 76.5% (cut-off point, 0.38; Table 7). These values are superior to those of serum CEA (sensitivity, 62.1%; specificity, 51.8%; cut-off point, 0.38). When serum sHB-EGF was combined with serum CEA, the sensitivity was slightly increased; the sensitivity was 77.3% and specificity was 76.5% (cut-off point, 0.38), respectively. When serum sHB-EGF was combined with serum CEA, the sensitivity was slightly increased; the sensitivity and specificity were 77.3% and 76.5% (cut-off point, 0.38). Collectively, serum sHB-EGF exhibited a remarkable diagnostic accuracy to predict GC both as a single biomarker and as a part of multiple-markers panel in GC (Table 7).
Table 7.
Logistic regression determination of the diagnostic accuracy of serum soluble heparin-binding epidermal growth factor-like growth factor compared with those of serum carcinoembryonic antigen for prediction of gastric cancer
| Markers1 | Cut-off point2 | Sensitivity | Specificity |
| Serum sHB-EGF | 0.38 | 76.1% | 76.5% |
| Serum CEA | 0.38 | 62.1% | 51.8% |
| Serum sHB-EGF + CEA | 0.38 | 77.3% | 76.5% |
Each marker is included as a linear term and evaluated as a panel from one to two markers combination.
Cut-off point means the probability cut-off point to classify subjects as having gastric cancer (GC) or not in binary logistic regression. CEA: Carcinoembryonic antigen; sHB-EGF: Soluble heparin-binding epidermal growth factor-like growth factor.
DISCUSSION
Increased EGFR levels are associated with poor prognosis in patients with GC[10,24]. HB-EGF, a ligand of the EGFR family, is initially synthesized as a pro-HB-EGF, a membrane-bound precursor form. It is later released into circulation as a soluble, mature form[15]. This sHB-EGF activates EGFR and acts as a potent growth factor[16-19]. HB-EGF is a critical molecular component of many normal physiological processes[14]. However, uncontrolled HB-EGF expression is linked to tumor formation. Thus, HB-EGF may become a promising biomarker or treatment target for cancer. Several studies have shown that HB-EGF is overexpressed in GC tissues and GC cell lines[20], and overexpressed HB-EGF is correlated with far-advanced stage of GC[10]. However, there is little quantitative data demonstrating the clinical significance of serum sHB-EGF in relation to GC tumorigenesis and progression such as TNM stage. There is also little known about the usefulness of this soluble factor as a biomarker for GC. In this study, we gathered quantitative information about the clinical significance of serum sHB-EGF levels in GC and validated serum sHB-EGF as a useful and reliable serological biomarker for GC.
We divided the subjects into 4 disease groups based on the theory of gastric carcinogenesis (gastritis-dysplasia-carcinoma)[21]: normal mucosa/gastritis (control), IM/dysplasia (high-risk), EGC, and AGC. Control group included subjects with normal gastric mucosa, simple CSG, and CAG because these patients have a relatively lower risk of GC development compared with IM/dysplasia. We did not subdivide patients into IM and dysplasia (adenoma) in the high-risk group because the number of subjects in each group was too small to be determined statistically significant. We divided the cancer patients into EGC and AGC groups because the prognosis is definitively different between EGC and AGC[1,3]. Interestingly, we observed that serum sHB-EGF levels increased along the carcinogenic sequence, although there was no statistically significant difference between the high-risk and control groups (Table 2). Serum sHB-EGF levels were also not significantly different between CAG and CSG groups or between CSG and normal mucosa groups in the control group (Table 3). However, significant differences were observed between the EGC and control groups, or the EGC and high-risk groups, respectively (post-hoc Bonferroni, all P < 0.05). Serum sHB-EGF levels were also significantly higher in AGC group compared with the other groups (post-hoc Bonferroni, all P < 0.001). This result suggests that circulating sHB-EGF plays an important role in GC tumorigenesis and progression, and it is valuable to investigate the usefulness of serum sHB-EGF as a serological biomarker or treatment target for GC.
To validate serum sHB-EGF as a desirable serum biomarker to predict the presence of GC, we generated ROC curves and calculated AUC values. We also performed logistic regression analysis to determine the best sensitivity and specificity for prediction of GC (Figure 1 and Table 7). We compared the results from sHB-EGF with the results from CEA, a well-known gastrointestinal tumor biomarker. Sensitivity/specificity of serum CEA for detection of GC were only around 50%-60% (Table 7), consistent with other previous studies[5,25]. However, the sensitivity and specificity of serum sHB-EGF were both greater than 75% (Table 7). When serum sHB-EGF was combined with serum CEA, the sensitivity was slightly elevated (76.1%→77.3%, Table 6). These are notable results compared with previous GC biomarker studies[5,25-27].
Clinicopathologically, serum sHB-EGF levels were closely correlated with depth of invasion, lymph node metastasis, distant metastasis, and primary tumor size (Table 5). This implies that sHB-EGF is involved not only in GC tumorigenesis, but also in GC expansion, invasion, and metastasis. This result is consistent with previous studies[10,11]. To our knowledge, this is the first study to evaluate serum sHB-EGF levels quantitatively according to the gastric carcinogenic sequence, to analyze the correlations between serum sHB-EGF and clinicopathological features of GC, such as TNM stage, and to validate serum sHB-EGF as a desirable serological biomarker for GC.
Previous studies reported that sHB-EGF levels were influenced by H. pylori infection[11]. However, in our study, serum sHB-EGF levels were not correlated with the status of H. pylori infection (Table 5). This discrepancy may be originated from the differences in the genetic background of enrolled subjects or different strains of H. pylori between the two studies because variation in the clinical presentation of H. pylori infection is attributable to strain diversity and host susceptibility[28,29]. However, we did not study about this in the current study.
A previous study showed that the activity of pro-HB-EGF was higher in intestinal type of GC compared with diffuse type of GC[13]. However, the relationship between the activity of sHB-EGF levels and histological differentiation has not been yet evaluated in previous studies. In this study, we observed that serum sHB-EGF levels tend to be higher in diffuse type than intestinal type of GC although it was not statistically significant (P = 0.078, Table 5). A study group reported that sHB-EGF promotes peritoneal carcinomatosis in patients with GC[10]. Peritoneal carcinomatosis occurs frequently in patients with diffuse scirrhous type of GC. These past reports support our current results. However, to confirm this, a further study may be necessary in the future.
We also observed that serum sHB-EGF levels were inversely correlated with age in GC patients (Table 5), whereas this value was not affected by age in non-cancer groups (Table 6), which implies that age itself may not affect the serum levels of sHB-EGF. Rather, higher levels of serum sHB-EGF in relatively younger GC patients than older patients may suggest that serum sHB-EGF may contribute to GC carcinogenesis especially in young age. However, we cannot currently explain the underlying mechanism of this phenomenon.
One of limitations of this study is the relatively small sample sizes, although statistical power of the current sample size of each group was > 80%. Additionally, we did not evaluate the relationship between serum sHB-EGF levels and prognosis of GC patients by directly comparing overall survival because the observation period was too short to evaluate the survival of the patients with GC. However, Table 5 showing the close correlations between high-levels of serum sHB-EGF and the presence lymph node and distant metastasis may support the correlation between high-levels of serum sHB-EGF and poor prognosis of GC indirectly because these two factors are the most important prognostic indicators for GC patients[30-32].
In conclusion, in this study, we evaluated the clinical significance of serum sHB-EGF levels in GC and validated serum sHB-EGF as a promising diagnostic and prognostic biomarker for GC. Our results also provide a rationale for blockade of sHB-EGF as a promising effective treatment target for GC, especially for advanced GC. Actually, several past studies have shown a remarkable antitumor effect of an HB-EGF inhibitor alone or in combination with various anticancer agents in cancer including GC[12,33]. To confirm this, we will conduct a large-scaled study in the future.
COMMENTS
Background
Early detection of gastric cancer (GC) is the most important clinical issue. Although endoscopic examination is an ideal, highly reliable technique for early detection of GC, it has some limitations as a routine screening method because of the risk associated with invasive procedure. Therefore, identification of inexpensive, reliable and less-invasive serum biomarkers is a great clinical challenge. However, research is still underway to identify effective serum biomarkers for GC.
Research frontiers
Heparin-binding epidermal growth factor-like growth factor (HB-EGF) has been thought to be associated with GC development and progression, and demonstrated to be overexpressed in human GC tissues. Because HB-EGF can be released into circulation as a mature soluble form of HB-EGF (sHB-EGF), it can be measured in serum and can be used as a serum biomarker for GC. The authors determined how serum levels of sHB-EGF related to the ‘gastritis-dysplasia-carcinoma’ sequence of gastric carcinogenesis and validated its usefulness as a biomarker for GC compared with serum CEA, a classic biomarker for gastrointestinal tumors.
Innovations and breakthroughs
Recent reports showed that increased epidermal growth factor receptor levels are associated with poor prognosis in patients with GC and HB-EGF expression is linked to tumor formation although HB-EGF is a critical molecular component of many normal physiological processes. Thus, HB-EGF may become a promising biomarker or treatment target for GC. Several studies have shown that HB-EGF is overexpressed in GC tissues, and overexpressed HB-EGF is correlated with far-advanced stage of GC. However, there is little quantitative data demonstrating the clinical significance of serum sHB-EGF in relation to GC tumorigenesis and progression such as TNM stage.
This is the first study to evaluate serum sHB-EGF levels quantitatively according to the gastric carcinogenic sequence, to analyze the correlations between serum sHB-EGF and clinicopathological features of GC, such as TNM stage, and to validate serum sHB-EGF as a desirable serological biomarker for GC.
Applications
The study results suggest that sHB-EGF are closely correlated with advanced TNM stage and higher in early gastric cancer (EGC) group than high-risk group, and higher in advanced gastric cancer group than EGC group. Additionally, this study demonstrated a remarkable diagnostic accuracy of serum sHB-EGF for GC.
Peer-review
This is an interesting manuscript with innovative endpoint and potential clinical impact. In this manuscript, authors studied the clinical usefulness of soluble heparin-binding EGF-like growth factor as a biomarker for GC. HB-EGF is overexpressed in several cancer cell lines and cancer tissues including human GC tissues. HB-EGF is also reported to be involved in malignant phenotype and chemo-resistance of cancer cells. Therefore this study would be useful to develop a useful biomarker for GC diagnosis.
Footnotes
Supported by Yonsei University College of Medicine for 2014, No. 3-2014-0115.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: June 2, 2014
First decision: July 21, 2014
Article in press: September 19, 2014
P- Reviewer: Antonakopoulos N, Mekada E S- Editor: Qi Y L- Editor: A E- Editor: Zhang DN
References
- 1.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 2.Hundahl SA, Phillips JL, Menck HR. The National Cancer Data Base Report on poor survival of U.S. gastric carcinoma patients treated with gastrectomy: Fifth Edition American Joint Committee on Cancer staging, proximal disease, and the “different disease” hypothesis. Cancer. 2000;88:921–932. [PubMed] [Google Scholar]
- 3.Nagata T, Ikeda M, Nakayama F. Changing state of gastric cancer in Japan. Histologic perspective of the past 76 years. Am J Surg. 1983;145:226–233. doi: 10.1016/0002-9610(83)90068-5. [DOI] [PubMed] [Google Scholar]
- 4.Kubota H, Kotoh T, Masunaga R, Dhar DK, Shibakita M, Tachibana M, Kohno H, Nagasue N. Impact of screening survey of gastric cancer on clinicopathological features and survival: retrospective study at a single institution. Surgery. 2000;128:41–47. doi: 10.1067/msy.2000.106812. [DOI] [PubMed] [Google Scholar]
- 5.Victorzon M, Haglund C, Lundin J, Roberts PJ. A prognostic value of CA 19-9 but not of CEA in patients with gastric cancer. Eur J Surg Oncol. 1995;21:379–384. doi: 10.1016/s0748-7983(95)92450-7. [DOI] [PubMed] [Google Scholar]
- 6.Salomon DS, Brandt R, Ciardiello F, Normanno N. Epidermal growth factor-related peptides and their receptors in human malignancies. Crit Rev Oncol Hematol. 1995;19:183–232. doi: 10.1016/1040-8428(94)00144-i. [DOI] [PubMed] [Google Scholar]
- 7.Piontek M, Hengels KJ, Porschen R, Strohmeyer G. Antiproliferative effect of tyrosine kinase inhibitors in epidermal growth factor-stimulated growth of human gastric cancer cells. Anticancer Res. 1993;13:2119–2123. [PubMed] [Google Scholar]
- 8.Kopp R, Ruge M, Rothbauer E, Cramer C, Kraemling HJ, Wiebeck B, Schildberg FW, Pfeiffer A. Impact of epidermal growth factor (EGF) radioreceptor analysis on long-term survival of gastric cancer patients. Anticancer Res. 2002;22:1161–1167. [PubMed] [Google Scholar]
- 9.García I, Vizoso F, Martín A, Sanz L, Abdel-Lah O, Raigoso P, García-Muñiz JL. Clinical significance of the epidermal growth factor receptor and HER2 receptor in resectable gastric cancer. Ann Surg Oncol. 2003;10:234–241. doi: 10.1245/aso.2003.05.010. [DOI] [PubMed] [Google Scholar]
- 10.Yasumoto K, Yamada T, Kawashima A, Wang W, Li Q, Donev IS, Tacheuchi S, Mouri H, Yamashita K, Ohtsubo K, et al. The EGFR ligands amphiregulin and heparin-binding egf-like growth factor promote peritoneal carcinomatosis in CXCR4-expressing gastric cancer. Clin Cancer Res. 2011;17:3619–3630. doi: 10.1158/1078-0432.CCR-10-2475. [DOI] [PubMed] [Google Scholar]
- 11.Yin Y, Grabowska AM, Clarke PA, Whelband E, Robinson K, Argent RH, Tobias A, Kumari R, Atherton JC, Watson SA. Helicobacter pylori potentiates epithelial: mesenchymal transition in gastric cancer: links to soluble HB-EGF, gastrin and matrix metalloproteinase-7. Gut. 2010;59:1037–1045. doi: 10.1136/gut.2009.199794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yotsumoto F, Yagi H, Suzuki SO, Oki E, Tsujioka H, Hachisuga T, Sonoda K, Kawarabayashi T, Mekada E, Miyamoto S. Validation of HB-EGF and amphiregulin as targets for human cancer therapy. Biochem Biophys Res Commun. 2008;365:555–561. doi: 10.1016/j.bbrc.2007.11.015. [DOI] [PubMed] [Google Scholar]
- 13.Murayama Y, Miyagawa J, Shinomura Y, Kanayama S, Isozaki K, Yamamori K, Mizuno H, Ishiguro S, Kiyohara T, Miyazaki Y, et al. Significance of the association between heparin-binding epidermal growth factor-like growth factor and CD9 in human gastric cancer. Int J Cancer. 2002;98:505–513. doi: 10.1002/ijc.10198. [DOI] [PubMed] [Google Scholar]
- 14.Higashiyama S, Abraham JA, Miller J, Fiddes JC, Klagsbrun M. A heparin-binding growth factor secreted by macrophage-like cells that is related to EGF. Science. 1991;251:936–939. doi: 10.1126/science.1840698. [DOI] [PubMed] [Google Scholar]
- 15.Higashiyama S, Lau K, Besner GE, Abraham JA, Klagsbrun M. Structure of heparin-binding EGF-like growth factor. Multiple forms, primary structure, and glycosylation of the mature protein. J Biol Chem. 1992;267:6205–6212. [PubMed] [Google Scholar]
- 16.Miyata K, Yotsumoto F, Nam SO, Kuroki M, Miyamoto S. Regulatory mechanisms of the HB-EGF autocrine loop in inflammation, homeostasis, development and cancer. Anticancer Res. 2012;32:2347–2352. [PubMed] [Google Scholar]
- 17.Ito N, Kawata S, Tamura S, Kiso S, Tsushima H, Damm D, Abraham JA, Higashiyama S, Taniguchi N, Matsuzawa Y. Heparin-binding EGF-like growth factor is a potent mitogen for rat hepatocytes. Biochem Biophys Res Commun. 1994;198:25–31. doi: 10.1006/bbrc.1994.1004. [DOI] [PubMed] [Google Scholar]
- 18.Hashimoto K, Higashiyama S, Asada H, Hashimura E, Kobayashi T, Sudo K, Nakagawa T, Damm D, Yoshikawa K, Taniguchi N. Heparin-binding epidermal growth factor-like growth factor is an autocrine growth factor for human keratinocytes. J Biol Chem. 1994;269:20060–20066. [PubMed] [Google Scholar]
- 19.Miyazaki Y, Shinomura Y, Higashiyama S, Kanayama S, Higashimoto Y, Tsutsui S, Zushi S, Taniguchi N, Matsuzawa Y. Heparin-binding EGF-like growth factor is an autocrine growth factor for rat gastric epithelial cells. Biochem Biophys Res Commun. 1996;223:36–41. doi: 10.1006/bbrc.1996.0842. [DOI] [PubMed] [Google Scholar]
- 20.Naef M, Yokoyama M, Friess H, Büchler MW, Korc M. Co-expression of heparin-binding EGF-like growth factor and related peptides in human gastric carcinoma. Int J Cancer. 1996;66:315–321. doi: 10.1002/(SICI)1097-0215(19960503)66:3<315::AID-IJC8>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 21.Correa P. Human gastric carcinogenesis: a multistep and multifactorial process--First American Cancer Society Award Lecture on Cancer Epidemiology and Prevention. Cancer Res. 1992;52:6735–6740. [PubMed] [Google Scholar]
- 22.Reim D, Loos M, Vogl F, Novotny A, Schuster T, Langer R, Becker K, Höfler H, Siveke J, Bassermann F, et al. Prognostic implications of the seventh edition of the international union against cancer classification for patients with gastric cancer: the Western experience of patients treated in a single-center European institution. J Clin Oncol. 2013;31:263–271. doi: 10.1200/JCO.2012.44.4315. [DOI] [PubMed] [Google Scholar]
- 23.Dixon MF, Genta RM, Yardley JH, Correa P. Classification and grading of gastritis. The updated Sydney System. International Workshop on the Histopathology of Gastritis, Houston 1994. Am J Surg Pathol. 1996;20:1161–1181. doi: 10.1097/00000478-199610000-00001. [DOI] [PubMed] [Google Scholar]
- 24.Nicholson RI, Gee JM, Harper ME. EGFR and cancer prognosis. Eur J Cancer. 2001;37 Suppl 4:S9–15. doi: 10.1016/s0959-8049(01)00231-3. [DOI] [PubMed] [Google Scholar]
- 25.Chung HW, Kim JW, Lee JH, Song SY, Chung JB, Kwon OH, Lim JB. Comparison of the validity of three biomarkers for gastric cancer screening: carcinoembryonic antigen, pepsinogens, and high sensitive C-reactive protein. J Clin Gastroenterol. 2009;43:19–26. doi: 10.1097/MCG.0b013e318135427c. [DOI] [PubMed] [Google Scholar]
- 26.Nakopoulou L, Zinozi M, Theodoropoulos G, Papacharalampous N. Carcinoembryonic antigen detection by immunocytochemical methods in carcinomas of the colon and stomach. Dis Colon Rectum. 1983;26:269–274. doi: 10.1007/BF02562496. [DOI] [PubMed] [Google Scholar]
- 27.Huang Z, Zhang X, Lu H, Wu L, Wang D, Zhang Q, Ding H. Serum trefoil factor 3 is a promising non-invasive biomarker for gastric cancer screening: a monocentric cohort study in China. BMC Gastroenterol. 2014;14:74. doi: 10.1186/1471-230X-14-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Park SM, Hong SI, Jung HY, Yang SK, Kim HR, Min YI, Hong WS. Antigenic diversity and serotypes of Helicobacter pylori associated with peptic ulcer diseases. Korean J Intern Med. 1998;13:104–109. doi: 10.3904/kjim.1998.13.2.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shortridge VD, Stone GG, Flamm RK, Beyer J, Versalovic J, Graham DW, Tanaka SK. Molecular typing of Helicobacter pylori isolates from a multicenter U.S. clinical trial by ureC restriction fragment length polymorphism. J Clin Microbiol. 1997;35:471–473. doi: 10.1128/jcm.35.2.471-473.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen CY, Wu CW, Lo SS, Hsieh MC, Lui WY, Shen KH. Peritoneal carcinomatosis and lymph node metastasis are prognostic indicators in patients with Borrmann type IV gastric carcinoma. Hepatogastroenterology. 2002;49:874–877. [PubMed] [Google Scholar]
- 31.Ichikura T, Tomimatsu S, Okusa Y, Uefuji K, Tamakuma S. Comparison of the prognostic significance between the number of metastatic lymph nodes and nodal stage based on their location in patients with gastric cancer. J Clin Oncol. 1993;11:1894–1900. doi: 10.1200/JCO.1993.11.10.1894. [DOI] [PubMed] [Google Scholar]
- 32.Hochwald SN, Kim S, Klimstra DS, Brennan MF, Karpeh MS. Analysis of 154 actual five-year survivors of gastric cancer. J Gastrointest Surg. 2000;4:520–525. doi: 10.1016/s1091-255x(00)80095-5. [DOI] [PubMed] [Google Scholar]
- 33.Sanui A, Yotsumoto F, Tsujioka H, Fukami T, Horiuchi S, Shirota K, Yoshizato T, Kawarabayashi T, Kuroki M, Miyamoto S. HB-EGF inhibition in combination with various anticancer agents enhances its antitumor effects in gastric cancer. Anticancer Res. 2010;30:3143–3149. [PubMed] [Google Scholar]


