Fig. 2.
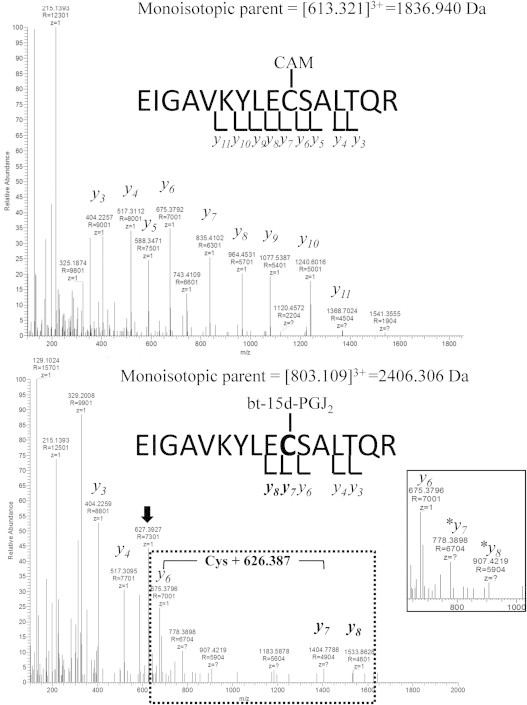
Mass spectrometric identification of C157 as a site of rRac1 modification. rRac1 was reacted with a 1:1 molar ratio of bt-15d-PGJ2:rRac1 for 1 h at room temperature. Reaction mixtures were alkylated (carboxyamidomethylated) and subjected to tryptic digestion, separated via HPLC and detected and analyzed via high resolution mass spectrometry with data dependent selection of m/z for tandem mass spectrometry. The rRac1 peptide containing C157, EIGAVKYLECSALTQR, was observed in both an alkylated form (top spectrum) and an adducted bt-15d-PGJ2-modified form (bottom spectrum). The top panel shows the alkylated (carboxyamidomethylated; CAM) peptide indicated by a series of y-ions. The CAM adduct is indicated on C157. The bottom panel shows bt-15d-PGJ2-adducted peptide indicated by a series of y-ions, showing the mass gap of Cys+626.387 Da (bt-15d-PGJ2) within the dotted box. The inset contains a close-up of the region of the m/z spectrum from 650 to 1000. ⁎y7 and ⁎y8 indicate the absence of the Cys+626.387 Da mass gap, and the black arrow indicates the m/z of 627.393, consistent with [bt-15d-PGJ2]+1, as described in Results.
