Abstract
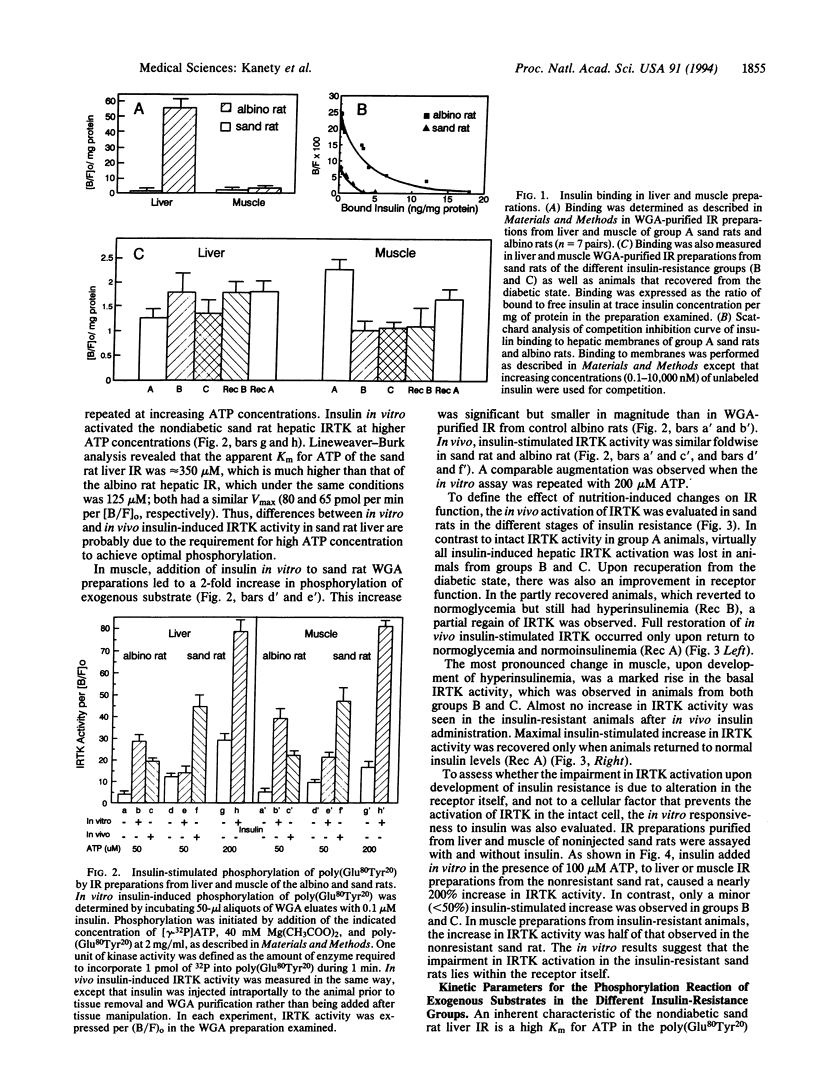
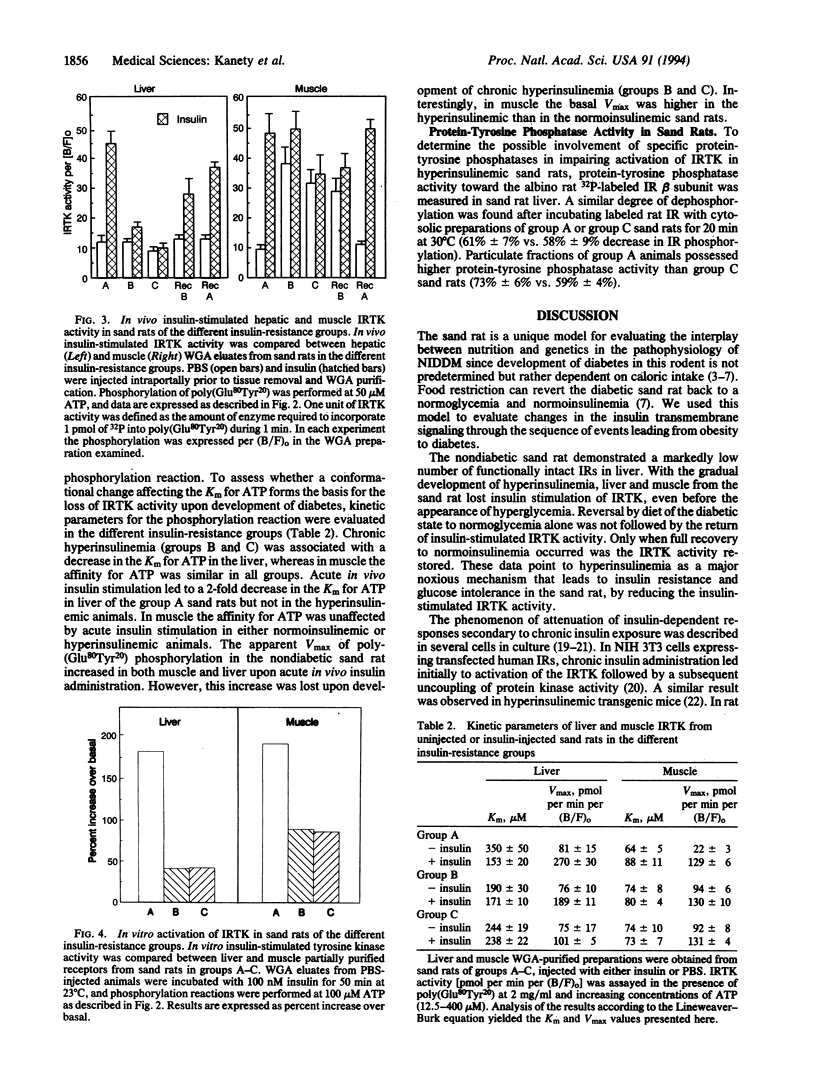
The insulin receptor was evaluated at different disease stages in the sand rat (Psammomys obesus), a model for nutrition-induced diabetes. Nondiabetic sand rats showed markedly low receptor number in liver compared with albino rats. Their receptor had an intact tyrosine kinase activity but a higher Km for ATP in the phosphorylation reaction of exogenous substrates. The initial effects of overeating (i.e., development of hyperinsulinemia without hyperglycemia) were associated in the sand rat with a dramatic decrease in in vitro and in vivo insulin-induced receptor tyrosine kinase activity in both liver and muscle. In muscle, this coincided with a decrease in receptor number and an increase in basal tyrosine kinase activity. Similar changes were observed upon development of hyperinsulinemia with hyperglycemia. Upon recovery from the diabetic state by diet restriction, the impaired receptor kinase activation was corrected. Complete restoration occurred only in animals that fully recovered from the diabetic state and became normoinsulinemic. These observations indicate that loss and gain of receptor tyrosine kinase activity were dependent on insulin levels. Thus, overeating may lead to the development of hyperinsulinemia through ineffective extraction of excess insulin by the scarce liver receptors. Hyperinsulinemia, in turn, causes a reversible reduction in receptor kinase activity, leading to insulin resistance. This sequence of events may be relevant to diet-related changes in human non-insulin-dependent diabetes mellitus.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler J. H., Lazarovici G., Marton M., Levy E. The diabetic response of weanling sand rats (Psammomys obesus) to diets containing different concentrations of salt bush (Atriplex halimus). Diabetes Res. 1986 Mar;3(3):169–171. [PubMed] [Google Scholar]
- Arner P., Pollare T., Lithell H., Livingston J. N. Defective insulin receptor tyrosine kinase in human skeletal muscle in obesity and type 2 (non-insulin-dependent) diabetes mellitus. Diabetologia. 1987 Jun;30(6):437–440. doi: 10.1007/BF00292549. [DOI] [PubMed] [Google Scholar]
- Arsenis G., Livingston J. N. Alterations in the tyrosine kinase activity of the insulin receptor produced by in vitro hyperinsulinemia. J Biol Chem. 1986 Jan 5;261(1):147–153. [PubMed] [Google Scholar]
- Boulton T. G., Nye S. H., Robbins D. J., Ip N. Y., Radziejewska E., Morgenbesser S. D., DePinho R. A., Panayotatos N., Cobb M. H., Yancopoulos G. D. ERKs: a family of protein-serine/threonine kinases that are activated and tyrosine phosphorylated in response to insulin and NGF. Cell. 1991 May 17;65(4):663–675. doi: 10.1016/0092-8674(91)90098-j. [DOI] [PubMed] [Google Scholar]
- Brillon D. J., Henry R. R., Klein H. H., Olefsky J. M., Freidenberg G. R. Functional and structural differences in human and rat-derived insulin receptors: characterization of the beta-subunit kinase activity. Endocrinology. 1988 Oct;123(4):1837–1847. doi: 10.1210/endo-123-4-1837. [DOI] [PubMed] [Google Scholar]
- Caro J. F., Ittoop O., Pories W. J., Meelheim D., Flickinger E. G., Thomas F., Jenquin M., Silverman J. F., Khazanie P. G., Sinha M. K. Studies on the mechanism of insulin resistance in the liver from humans with noninsulin-dependent diabetes. Insulin action and binding in isolated hepatocytes, insulin receptor structure, and kinase activity. J Clin Invest. 1986 Jul;78(1):249–258. doi: 10.1172/JCI112558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson M. B., Harris M. D., Rosenberg C. S. Inverse relationship of metabolic clearance rate of insulin to body mass index. Metabolism. 1987 Mar;36(3):219–222. doi: 10.1016/0026-0495(87)90179-x. [DOI] [PubMed] [Google Scholar]
- Faber O. K., Christensen K., Kehlet H., Madsbad S., Binder C. Decreased insulin removal contributes to hyperinsulinemia in obesity. J Clin Endocrinol Metab. 1981 Sep;53(3):618–621. doi: 10.1210/jcem-53-3-618. [DOI] [PubMed] [Google Scholar]
- Fiedler H., Schäfer H., Köhler E., Knospe S., Hahn H. J., Ziegler B., Gottschling H. D., Heinke P. Insulin and glucagon secretion and the insulin sensitivity of peripheric organs of colony-bred sand rats fed with pellet diet after weaning. Endokrinologie. 1977;70(3):301–320. [PubMed] [Google Scholar]
- Freidenberg G. R., Henry R. R., Klein H. H., Reichart D. R., Olefsky J. M. Decreased kinase activity of insulin receptors from adipocytes of non-insulin-dependent diabetic subjects. J Clin Invest. 1987 Jan;79(1):240–250. doi: 10.1172/JCI112789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freidenberg G. R., Reichart D., Olefsky J. M., Henry R. R. Reversibility of defective adipocyte insulin receptor kinase activity in non-insulin-dependent diabetes mellitus. Effect of weight loss. J Clin Invest. 1988 Oct;82(4):1398–1406. doi: 10.1172/JCI113744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutman A., Andreus A., Adler J. H. Hyperinsulinemia, insulin resistance and cataract formation in sand rats. Isr J Med Sci. 1975 Jul;11(7):714–722. [PubMed] [Google Scholar]
- HAINES H., HACKEL D. B., SCHMIDT-NIELSEN K. EXPERIMENTAL DIABETES MELLITUS INDUCED BY DIET IN THE SAND RAT. Am J Physiol. 1965 Feb;208:297–300. doi: 10.1152/ajplegacy.1965.208.2.297. [DOI] [PubMed] [Google Scholar]
- Kalderon B., Gutman A., Levy E., Shafrir E., Adler J. H. Characterization of stages in development of obesity-diabetes syndrome in sand rat (Psammomys obesus). Diabetes. 1986 Jun;35(6):717–724. doi: 10.2337/diab.35.6.717. [DOI] [PubMed] [Google Scholar]
- Karasik A., Rothenberg P. L., Yamada K., White M. F., Kahn C. R. Increased protein kinase C activity is linked to reduced insulin receptor autophosphorylation in liver of starved rats. J Biol Chem. 1990 Jun 25;265(18):10226–10231. [PubMed] [Google Scholar]
- Kasayama S., Yoshimura M., Oka T. Decreased expression of hepatic epidermal growth factor receptor gene in diabetic mice. J Mol Endocrinol. 1989 Jul;3(1):49–56. doi: 10.1677/jme.0.0030049. [DOI] [PubMed] [Google Scholar]
- King M. J., Sale G. J. Insulin-receptor phosphotyrosyl-protein phosphatases. Biochem J. 1988 Dec 15;256(3):893–902. doi: 10.1042/bj2560893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerner R. A., Green N., Alexander H., Liu F. T., Sutcliffe J. G., Shinnick T. M. Chemically synthesized peptides predicted from the nucleotide sequence of the hepatitis B virus genome elicit antibodies reactive with the native envelope protein of Dane particles. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3403–3407. doi: 10.1073/pnas.78.6.3403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miki E., Like A. A., Steinke J., Soeldner J. S. Diabetic syndrome in sand rats. II. Variability and association with diet. Diabetologia. 1967 Apr;3(2):135–139. doi: 10.1007/BF01222190. [DOI] [PubMed] [Google Scholar]
- Nadiv O., Cohen O., Zick Y. Defects of insulin's signal transduction in old rat livers. Endocrinology. 1992 Mar;130(3):1515–1524. doi: 10.1210/endo.130.3.1311243. [DOI] [PubMed] [Google Scholar]
- Reaven G. M. Insulin-independent diabetes mellitus: metabolic characteristics. Metabolism. 1980 May;29(5):445–454. doi: 10.1016/0026-0495(80)90170-5. [DOI] [PubMed] [Google Scholar]
- Rosen O. M. After insulin binds. Science. 1987 Sep 18;237(4821):1452–1458. doi: 10.1126/science.2442814. [DOI] [PubMed] [Google Scholar]
- Takayama S., White M. F., Kahn C. R. Phorbol ester-induced serine phosphorylation of the insulin receptor decreases its tyrosine kinase activity. J Biol Chem. 1988 Mar 5;263(7):3440–3447. [PubMed] [Google Scholar]
- Treadway J. L., Whittaker J., Pessin J. E. Regulation of the insulin receptor kinase by hyperinsulinism. J Biol Chem. 1989 Sep 5;264(25):15136–15143. [PubMed] [Google Scholar]
- Weir G. C. Non-insulin-dependent diabetes mellitus: interplay between B-cell inadequacy and insulin resistance. Am J Med. 1982 Oct;73(4):461–464. doi: 10.1016/0002-9343(82)90321-7. [DOI] [PubMed] [Google Scholar]
- Williams J. F., Olefsky J. M. Defective insulin receptor function in down-regulated HepG2 cells. Endocrinology. 1990 Oct;127(4):1706–1717. doi: 10.1210/endo-127-4-1706. [DOI] [PubMed] [Google Scholar]
- Zick Y. The insulin receptor: structure and function. Crit Rev Biochem Mol Biol. 1989;24(3):217–269. doi: 10.3109/10409238909082554. [DOI] [PubMed] [Google Scholar]
- Zick Y., Whittaker J., Roth J. Insulin stimulated phosphorylation of its own receptor. Activation of a tyrosine-specific protein kinase that is tightly associated with the receptor. J Biol Chem. 1983 Mar 25;258(6):3431–3434. [PubMed] [Google Scholar]


