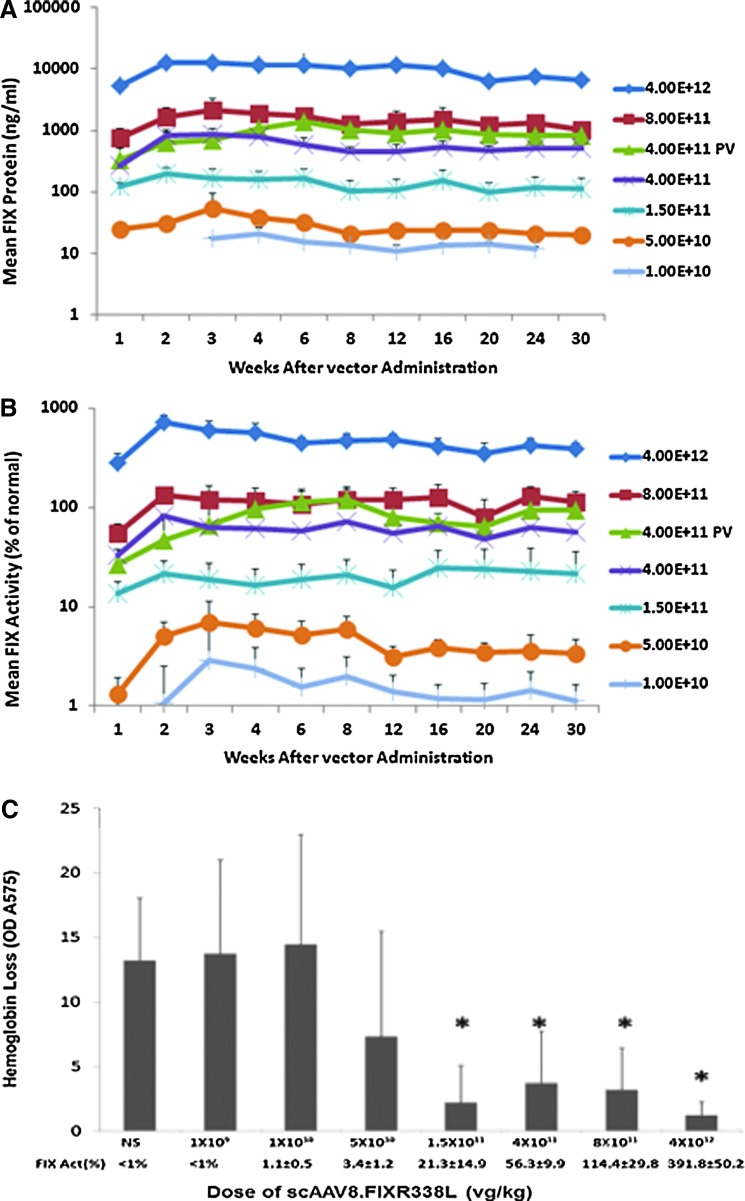
FIG. 2.
Dose response after scAAV8.FIXR338L vector was delivered by peripheral (tail) vein. FIX–/– mice (six to eight per group) received the indicated doses of scAAV8.FIXR338L as a single tail vein injection to model the peripheral venous route of dosing planned for human clinical application. So as to allow comparison of tail vein (TV) and portal vein (PV) administration using the same vector dosing examined in Fig. 1, separate groups of age-, weight-, and sex-matched FIX–/– mice received 4.0×1011 VG/kg by PV, with factor IX expression displayed by the green triangles. (A) Circulating factor IX protein. (B) Circulating factor IX activity. At any given dose, the specific activity of plasma factor IX (factor IX activity/factor IX antigen, expressed in units/mg protein) was five to eight times normal, with factor IX expression persisting over months. Plasma was examined at each time point for the development of antibodies directed against factor IX. No factor IX inhibitors (as measured by Bethesda assay) or anti-FIX IgG (as measured by ELISA) was detected in any animal throughout the observation. (C) Dose-dependent protection from hemorrhage in the tail transection bleeding challenge. At 32 weeks after vector delivery, all mice from each dose group had plasma factor IX activity determined (as recorded on the x axis below each column) and then underwent a tail transection bleeding challenge and the amount of blood loss in 10 min after wounding was collected and quantitated by measuring the optical density (OD) of the shed hemoglobin.