Figure 2.
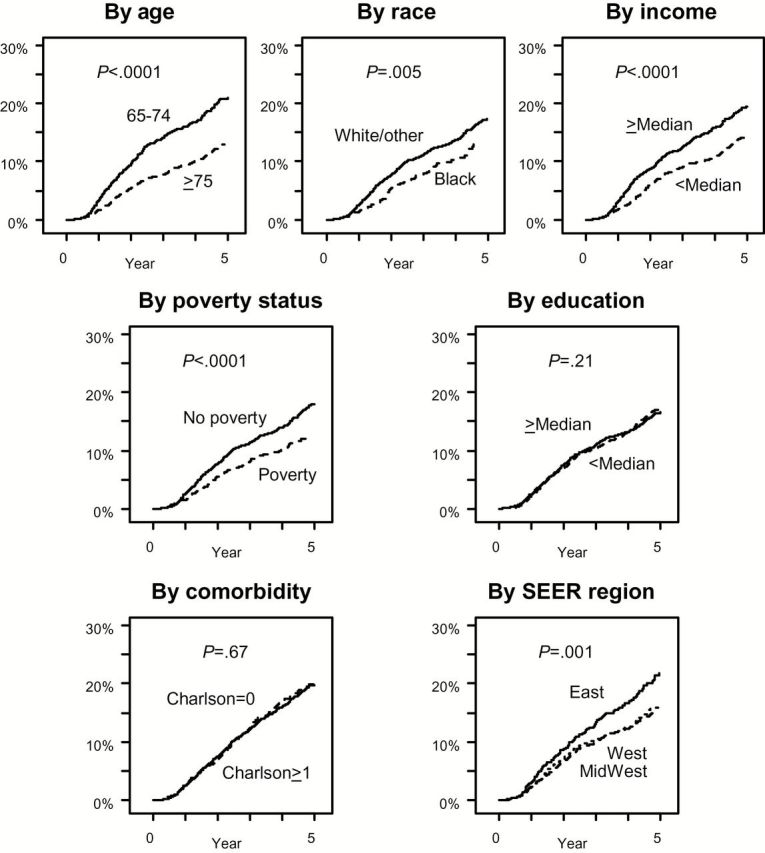
Five-year cumulative incidence of docetaxel use from diagnosis among patients presenting with metastatic prostate cancer by socioeconomic status, demographic, comorbidity, and geographic factors. (Cumulative incidence by ethnicity was not analyzed because of the small subset of Hispanic patients.) Patients for all years of diagnosis were included. Five-year cumulative incidence estimates by factor were: by age: 24.8% (95% confidence interval [CI] = 22.7% to 26.9%) for patients age 65 to 74 years vs 15.4% (95% CI = 13.7% to 17.1%) for patients older than 75 years; by race: 20.3% (95% CI = 18.9% to 21.8%) for white/other patients vs 16.9% (95% CI = 13.5% to 20.6%) for black patients; by income: 22.8% (95% CI = 20.8% to 24.8%) for patients from Census tract regions with incomes greater than the median vs 16.9% (95% CI = 15.2% to 18.8%) for patients from Census tract regions with incomes lower than the median; by poverty status: 21.2% (95% CI = 19.7% to 22.8%) for patients with no evidence of poverty and 14.5% (95% CI = 12.0% to 17.1%) for patients with evidence of poverty; by education: 20.1% (95% CI = 18.2% to 22.0%) for patients from Census tract regions with education over the median vs 19.8% (17.9% to 21.7%) for patients from Census tract regions with education under the median; by comorbidity status: 19.8% (18.2% to 21.3%) for patients with a Charlson score of 0 vs 20.1% (95% CI = 17.4% to 22.9%) for patients with a Charlson score over 1; by Surveillance, Epidemiology, and End Results Program (SEER) region: 25.6% (95% CI = 22.0% to 29.4%) for patients from Eastern regions vs 18.8% (95% CI = 16.9% to 20.8%) for patients from Western regions vs 18.5% (95% CI = 16.4% to 20.7%) for patients from Midwestern regions. All four statistically significant univariate predictors (age, race, income, and SEER region) remained statistically significant predictors (P < .01 for each) in adjusted multivariable regression. All statistical tests were two-sided. SEER = Surveillance, Epidemiology, and End Results Program.
