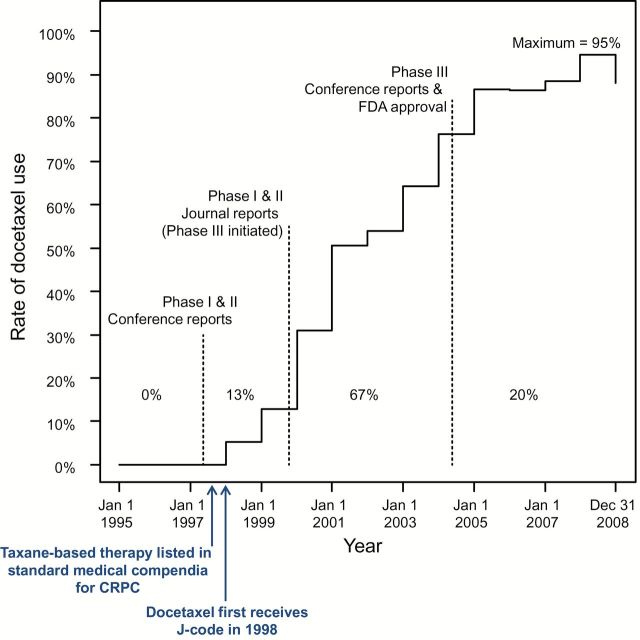
Figure 3.
Proportion using docetaxel over time with landmark events. Thirteen percent of total (ie, maximum) diffusion occurred prior to phase I and II journal reports, 67% between phase I and II journal reports and initial phase III conference reports and US Food and Drug Administration (FDA) approval, and 20% after the phase III reports/FDA approval. Importantly, taxane-based therapy was included in standard medical compendia (ie, National Comprehensive Cancer Network) as a treatment for castration-resistant prostate cancer as early as 1997, with docetaxel in particular listed prior to publication of phase III evidence and FDA approval (76,77). CRPC = castration-resistant prostate cancer; FDA = US Food and Drug Administration.