Figure 5.
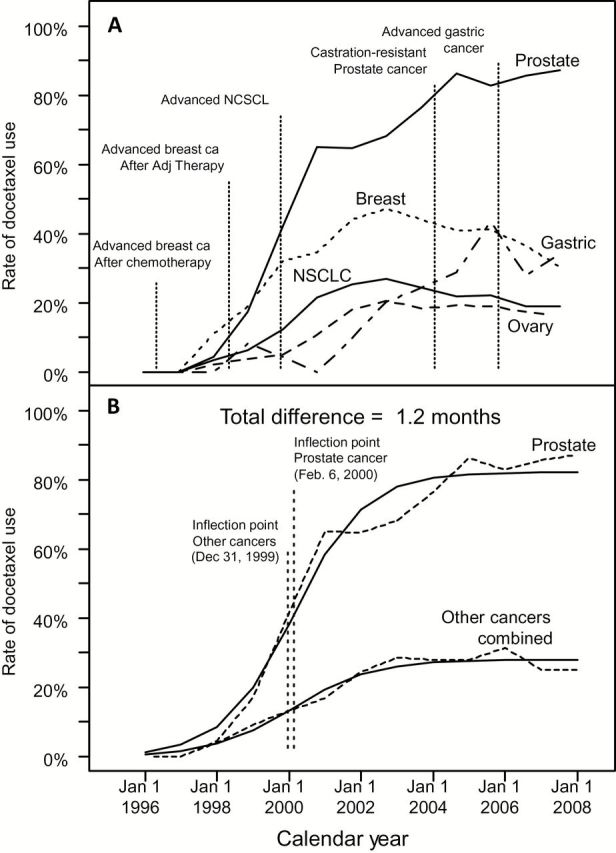
Rate of docetaxel use over time, by cancer type, with landmark events and model-fitted curves. A) The proportion using docetaxel over time for metastatic prostate cancer is compared with rates in advanced breast, lung, gastric, and ovarian cancers. US Food and Drug Administration (FDA) approval times for each cancer are shown. Docetaxel was approved for use in patients with locally advanced or metastatic breast cancer after failure of prior chemotherapy in May, 1996; in patients with metastatic breast cancer who failed adjuvant therapy in June, 1998; in patients with non–small cell lung cancer who failed cisplatin-based treatment in December, 1999; and in patients with advanced gastric cancer (in combination with 5-FU and cisplatin) in March, 2006. Docetaxel has not received an FDA indication for use in ovarian cancer patients, but is included to convey the similarity of diffusion patterns of docetaxel for a cancer in which prescriptions are strictly off-label. B) The observed proportions using docetaxel over time for metastatic prostate cancer are compared with the combined rates from advanced breast, lung, gastric, and ovarian cancers. Fitted model-based estimates are superimposed. The inflection points for the fitted curves indicate the time of maximum increase in the rate of docetaxel use and are approximately the same (1.2 months or 37 days difference) between the two curves. NSCLC = non–small cell lung cancer.
