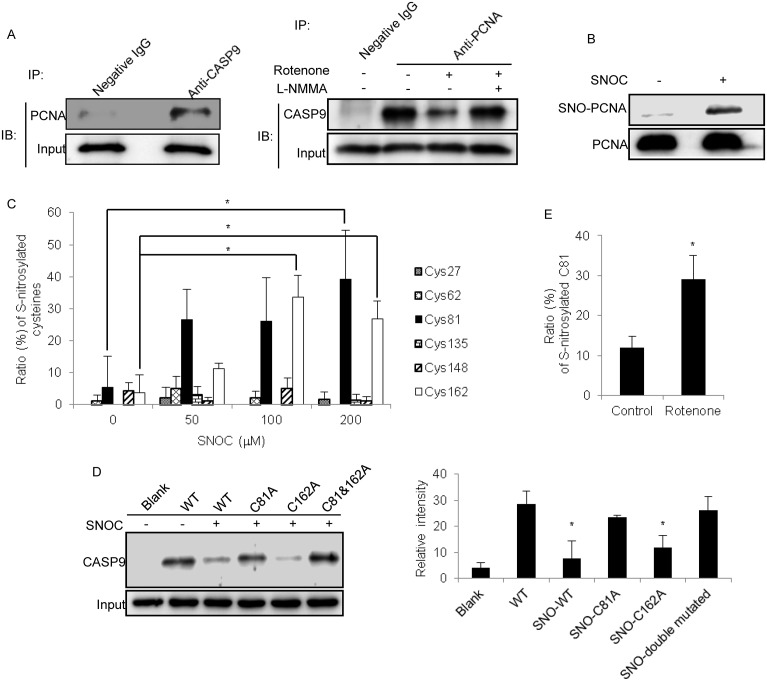
Fig 4. Effects of the S-nitrosylation status of PCNA on the interactions of PCNA and caspase-9.
(A) Diagonal CoIP using two antibodies against PCNA and caspase-9 in SH-SY5Y cells. The interactions of PCNA and caspase-9 were negatively correlated with the NO contents in SH-SY5Y cells. L-NMMA was used for the inhibition of nNOS, and rabbit IgG was used as a negative control for immunoprecipitation. (B) The S-nitrosylation of recombinant PCNA, identified by BST/Western blot, using SNOC as a NO donor. (C) Comparison of the sensitivity of the potential cysteine residues of recombinant PCNA to S-nitrosylation under different NO stress levels. The sensitivity of cysteine residues to NO modification is represented as the ratios of the S-nitrosylated peptides identified by LC-MS/MS to the sum of the corresponding peptides, which include all S-nitrosylated and non-S-nitrosylated peptides at certain sites (n = 3, *P<0.05). (D) Effects of the PCNA mutants under NO stress on the interactions of PCNA and caspase-9. In the pull-down experiment, the wild-type PCNA and three PCNA mutants, PCNA-C81A,-C162A and-C81A/C162A, were treated with SNOC and incubated with the HeLa cytosol, followed by enrichment with nickel-agarose beads and detection with Western blot using an antibody against caspase-9. The left panel shows the Western blot image, and the right panel presents the interaction of caspase-9 with different SNOC-modified recombinant PCNAs. The relative immune-recognition intensities were estimated based on the ratios of the specific band volume against the total band volumes for caspase-9 in the upper panel (n = 3, *P<0.05 versus WT PCNA). (E) Comparison of the S-nitrosylated status of PCNA at Cys81 in SH-SY5Y cells with and without rotenone treatment. The S-nitrosylation status of PCNA at Cys81 is represented as the ratios of the S-nitrosylated Cys81 peptide to the sum of the peptides that contained Cys81, which were identified by LC-MS/MS (n = 3, *P<0.05).