Abstract
Technologies based on RNA interference may be used for insect control. Sustainable strategies are needed to control vectors of Chagas disease such as Rhodnius prolixus. The insect microbiota can be modified to deliver molecules to the gut. Here, Escherichia coli HT115(DE3) expressing dsRNA for the Rhodnius heme-binding protein (RHBP) and for catalase (CAT) were fed to nymphs and adult triatomine stages. RHBP is an egg protein and CAT is an antioxidant enzyme expressed in all tissues by all developmental stages. The RNA interference effect was systemic and temporal. Concentrations of E. coli HT115(DE3) above 3.35 × 107 CFU/mL produced a significant RHBP and CAT gene knockdown in nymphs and adults. RHBP expression in the fat body was reduced by 99% three days after feeding, returning to normal levels 10 days after feeding. CAT expression was reduced by 99% and 96% in the ovary and the posterior midgut, respectively, five days after ingestion. Mortality rates increased by 24-30% in first instars fed RHBP and CAT bacteria. Molting rates were reduced by 100% in first instars and 80% in third instars fed bacteria producing RHBP or CAT dsRNA. Oviposition was reduced by 43% (RHBP) and 84% (CAT). Embryogenesis was arrested in 16% (RHBP) and 20% (CAT) of laid eggs. Feeding females 105 CFU/mL of the natural symbiont, Rhodococcus rhodnii, transformed to express RHBP-specific hairpin RNA reduced RHBP expression by 89% and reduced oviposition. Modifying the insect microbiota to induce systemic RNAi in R. prolixus may result in a paratransgenic strategy for sustainable vector control.
Author Summary
Rhodnius prolixus is an important vector of Chagas disease. The development of insecticide resistance in triatomines has raised the need for new control methods. We propose, as a proof-of-concept, the use of symbiotic bacteria expressing dsRNA in a paratransgenic approach to control vector-borne disease. We first show that ingestion of E. coli, producing long dsRNA specific for R. prolixus genes, can produce systemic RNAi in this insect. By targeting genes with antioxidant function (RHBP and catalase), we show that RNAi effects on nymphs and adult females are systemic and temporal, affecting development and fecundity. Finally, we show that the natural vector symbiont, R. rhodnii, also can be modified to induce systemic RNA interference. The E. coli system can serve to screen potential targets for development of a symbiont-based vector control product that then can be transferred to R. rhodnii.
Introduction
Oral delivery of dsRNA has been proposed as a method to control insect pests such as termites, fruit flies, flour beetles, pea aphids, tobacco hornworms and honeybees, among others [1–7]. These studies have shown variable efficacy of oral-mediated delivery, suggesting that each species and gene may behave differently. Paper impregnated with dsRNA was used effectively to silence genes in termites [7]. Plants coated with dsRNA, or genetically modified to express dsRNA specific for insect genes, also have been tested for the control of various plant pests [8], and bacteria have been used to deliver dsRNA in lepidopterans [9]. Studies on mosquito species that transmit diseases to humans showed effective oral delivery through exposure to dsRNA in solution [10] or stabilized in chitosan [11]. These studies suggest that oral delivery of dsRNA may provide a method to control disease vectors.
Rhodnius prolixus is an efficient vector of Trypanosoma cruzi, the etiological agent of Chagas disease, and is primarily controlled by domiciliary insecticide applications. Even though residual insecticide treatment inside houses is effective in decreasing the vector densities and minimizing the risk of infection [12], the sustainability of this method in the Central American and Andean regions is limited by the potential re-infestation by species from sylvatic habitats, the need for repeated applications of insecticides, and the potential development of resistance [13–15]. In 2002, the Tropical Disease Research Scientific and Technical Advisory Council and the World Health Assembly (resolution WHA 51.14) recommended that future research efforts focus on the development of control strategies for the local entomological conditions in these regions, where sylvatic vectors pose a serious threat to sustainability (http://www.who.int/tdr/diseases/chagas/strat_dir_chagas/en/index2.html#).
The use of genetically transformed insect symbiotic bacteria to block parasite transmission has been proposed as a sustainable control method for vector-borne diseases. The bacteria are modified to express an anti-parasite peptide to kill the parasite in the intestine of the vector [16–18]. Bacterial ingestion would allow introduction of transformed bacteria into a vector population [19]. Given that the vector is indirectly transformed through its symbionts, they are considered paratransgenic insects. Our group has worked for the past decade on the development of a paratransgenic model for transmission-blocking control for Chagas disease. The strategy is based on oral delivery of intestinal symbionts of R. prolixus in a paste-like formulation simulating triatomine feces [16, 19, 20]. R. prolixus harbors extracellular actinobacteria, Rhodococcus rhodnii, in the intestinal lumen [21]. The bacteria are shed in the feces and spread horizontally through the vector’s coprophagic habits [22]. Following a blood meal, R. rhodnii grows exponentially in the anterior midgut, the same compartment where T. cruzi establishes after entering the insect in an infected blood meal. Populations of R. rhodnii can reach 109 CFU per 5th instar nymph 10 days after the bloodmeal [23].
Coprophagous transmission and fast bacterial growth in the same location as the parasite allow oral transmission of transformed symbionts and production of antiparasitic molecules in the insect midgut. An adaptation of this strategy would be to transform the symbiotic bacteria to attack the vector’s physiology in addition to its vectorial competence. We propose that transformed gut symbiotic bacteria may be ingested by R. prolixus to produce dsRNA specific for genes important in insect development, fecundity and vectorial competence.
As a proof-of-concept, we used Escherichia coli [HT115(D3)][24] and R. rhodnii to deliver dsRNA specific for genes with antioxidant functions and involved in egg production and development: Rhodnius heme binding protein (RHBP) and catalase (CAT) [25–28]. Hematophagous insects are exposed to large amounts of free heme derived from the blood meal. Heme is a pro-oxidant molecule that stimulates lipid peroxidation by increasing reactive oxygen species (ROS) production [29]. Heme-RHBP is an egg protein that enters the developing oocytes through receptor-mediated endocytosis and is used as a heme source for embryogenesis [30–32]. It is secreted in all developmental stages by the fat body as a heme-bound protein and circulates in the hemolymph in apo and heme forms [33]. Catalase is a ubiquitous enzyme responsible for dismutation of hydrogen peroxide into molecular oxygen and water [34]. Catalase activity has been described in many tissues of R. prolixus [27] and plays a key role in the protection against oxidative damage [35]. Thus, RHBP and CAT physiologically protect cellular components against heme-induced oxidative damages. Knocking down RHBP expression affects the physiology and fecundity of females [30].
We proposed that oral delivery of bacteria expressing dsRNA for CAT or RHBP should affect the normal development of nymphs and female fecundity. Our results show that E. coli can express and deliver RHBP and CAT dsRNA into the midgut of R. prolixus, leading to the systemic spread of RNAi to the fat body, anterior and posterior midgut, and ovaries. Modified symbiotic R. rhodnii also were effective at inducing gene silencing with physiological effects. The potential application of this technology using bacterial symbionts for vector-borne disease control is discussed.
Methods
Insects
We used nymphs and adult stages of R. prolixus maintained at 28°C and 70% relative humidity. Conditions were maintained as described in S1 Text.
Ethics statement
All animal care and experimental protocols were conducted following the guidelines of the institutional care and use committee (Committee for Evaluation of Animal Use for Research from the Federal University of Rio de Janeiro, CAUAP-UFRJ) and the NIH Guide for the Care and Use of Laboratory Animals (ISBN 0–309–05377–3). The protocols were approved by CAUAP-UFRJ under registry #IBQM001. Technicians dedicated to the animal facility at the Institute of Medical Biochemistry (UFRJ) carried out all aspects related to rabbit husbandry under strict guidelines to ensure careful and consistent handling of the animals.
Plasmids and bacterial strains
Fragments from RHBP (375 bp) and CAT (453 bp) genes were cloned from R. prolixus cDNA into pGEM-T (Promega, Madison, Wisconsin, USA) plasmids to generate dsRNA, AINTEGUMENTA (ANT) gene from Arabidopsis thaliana was used as a control. Additionally, a RHBP and random nucleotide hairpin of ~100 bp were cloned into pBP2lac, an integration plasmid that integrates into the R. rhodnii chromosome (see S1 Text for details). All plasmids for dsRNA were purified and cloned into E. coli HT115(DE3) competent cells as described by Timmons [36] and plasmids with hairpins into R. rhodnii as described by Crampton et al [37]. PCR and transformation conditions are specified in SI text.
Bacterial culture and feeding conditions
E. coli HT115(DE3) containing the pGEM-T plasmid with inserts for RHBP, CAT, and the ANT gene, and without plasmid was grown in LB media containing ampicillin and/or tetracycline. After induction, the bacterial cells were harvested by centrifugation and a pellet was resuspended in fresh rabbit blood for artificial feeding. Each group was fed separately on individual feeding chambers containing blood. Three different concentrations of E. coli were used: 2.24 × 107 CFU/mL, 3.35 × 107 CFU/mL, and 5.4 × 107 CFU/mL. For the experiment with R. rhodnii, the bacteria containing the pBP2lac plasmid with inserts for RHBP and random nucleotides was grown in Brain Heart Infusion media containing kanamycin. For feeding, 4.8 × 105 CFU R. rhodnii/mL of blood was used.
Verification of knockdown at mRNA and protein level
The RNA from fat bodies, ovaries, posterior- and anterior- midguts of individual females was collected at different days post feeding. Gene expression was measured by cDNA synthesis and real time quantitative PCR (q-PCR). RNA from first and third stage nymphs was extracted from fat bodies and midgut. To evaluate apo-RHBP levels, hemolymph was collected from females. The binding of heme to RHBP was measured by progressively adding heme to 1 µl female hemolymph and recording the absorption spectra after each addition [33]. To evaluate CAT activity, anterior midguts from female adult insects were individually homogenized and used to measure CAT specific activity by spectrophotometry [27]. Midguts were also incubated with an oxidant-sensitive fluorophore dihydroethidium (DHE) and epifluorescence microscopy was performed. Experimental procedures are detailed in S1 Text.
Phenotype description
Adult females were maintained in individual chambers during the oviposition cycle and eggs were collected daily [38]. Ovaries were dissected and photographed. Nymphs were also observed daily to track mortality and molting.
Data analysis
ANOVA and Mann-Whitney tests were performed with the SPSS 17.0 software (IBM, Armonk, NY, USA) and Graph Pad Prisma (GraphPad Prism version 5.04 for Windows, GraphPad Software, La Jolla California USA, www.graphpad.com). All significant values have a p value less than 0.05.
Accession numbers
Rhodnius heme-binding protein GenBank: AF493801.1 Catalase Vector Base Transcript ID: RPRC007907_RA.
Results
Establishment of feeding conditions in females with RHBP construct
We amplified, cloned, sequenced, and expressed RHBP and CAT dsRNA in E. coli, a sequence from A. thaliana was used as a control gene. Production of dsRNA after IPTG induction was confirmed by purification of dsRNA of each specific bacterial culture. Gel electrophoresis showed the expected products of approximately 400 bp (S1A Fig.). Based on spectrophotometric analysis of triplicate extractions, each mL of culture at an OD600 0.8–1 had a median (interquartile range) of 1,542 (1,068–2,751) ng dsRNA for the RHBP E. coli strain and 1,944 (521–3,777) ng for the ANT E. coli strain. Insects were fed blood containing E. coli that expressed the dsRNA gene fragments, bacteria without plasmid, or blood free of bacteria. For the selection of appropriate concentrations of bacteria for the feeding, an “R” value was used to consider the amount of blood ingested in relation to the initial weight of each individual. Only females that engorged with >0.1 mL blood were included in the study. The amount of bacteria present in the blood showed no effect on R values even at the maximal bacterial concentration tested, 5.54 × 107 CFU/mL (S2A Fig.). Daily weight loss due to diuresis and defecation was monitored five days after feeding (S2B Fig.). There was no difference in weight loss between the groups fed concentrations of E. coli of 2.24 × 107, 3.35 × 107and 5.4 × 107CFU/mL. Individuals received at least 0.5–1.4 µg (5–14 ng dsRNA/µL blood) and 1–2.8 µg (10–28 ng dsRNA/µL blood) RHBP dsRNA at the lowest and highest bacterial concentrations, respectively.
Verification of RHBP and CAT knockdown in adult females
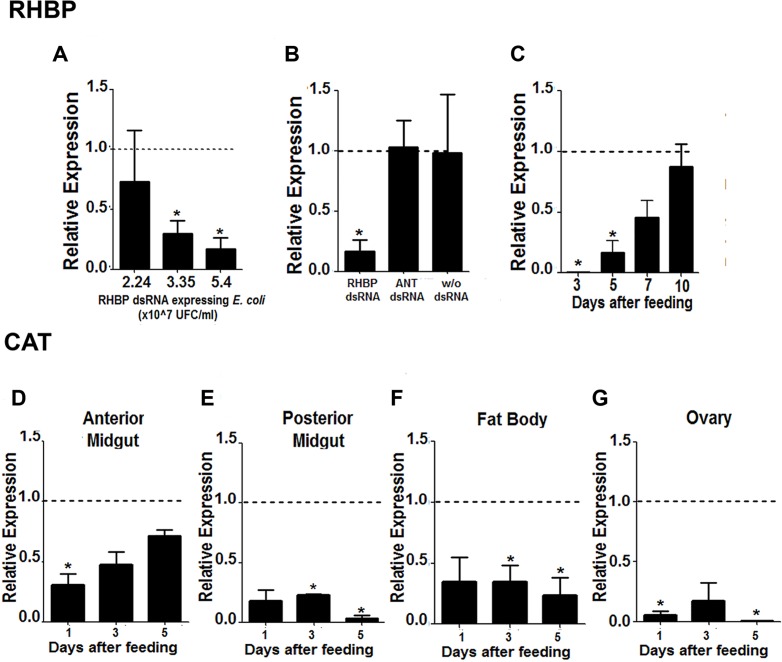
The RHBP gene expression in the fat body was measured by q-PCR in individuals fed E. coli producing RHBP or ANT dsRNA, relative to individuals fed blood alone. In adults, a significant (T-test, P< 0.05) reduction in RHBP expression was observed after feeding E. coli expressing RHBP dsRNA at 3.35 × 107 CFU/mL blood to 5.54 × 107 CFU/mL blood (Fig. 1A). Experimental groups fed bacteria expressing ANT dsRNA or bacteria alone presented no significant difference (Two way ANOVA, p > 0.05) from the sterile blood control (Fig. 1B). Protein and mRNA expression increase over the first six days after feeding, dropping afterwards to constitutive levels [25, 33]. In females fed E. coli expressing RHBP dsRNA at 5.54 × 107 CFU/mL blood, expression was significantly inhibited only on days three (99.6%) and five (85%) after feeding (T-test, P< 0.05) (Fig. 1C). Relative expression at day ten was significantly different from days three and five, suggesting temporal effects (ANOVA with Tukey post hoc test, P<0.05).
Figure 1. Dose- and time-dependent effect of RHBP knockdown, and tissue-dependent effect of CAT knock-down, in adult females.
Females were fed E. coli expressing dsRNA. RHBP (A) Expression of RHBP relative to actin on day five after feeding with different amounts of bacteria expressing RHBP dsRNA as compared to insects fed sterile blood: 2.24 × 107 CFU/ml of blood (n = 6), 3.35 × 107 CFU/ml of blood (n = 12) and 5.4 × 107 CFU/ml of blood (n = 8). (B) Expression of RHBP in insects fed 5.4 × 107 CFU/mL blood using bacteria expressing RHBP dsRNA, ANT dsRNA, and without dsRNA. Asterisk indicates statistically different values (T-test, P< 0.05) between experimental groups exposed to bacteria with RHBP dsRNA (n = 6), bacteria with ANT dsRNA (n = 8), bacteria without dsRNA (n = 6), as compared to groups fed blood alone (n = 6). (C) Time-dependent relative expression of RHBP in insects fed 5.4 × 107 CFU bacteria/ml blood ten days after feeding. Asterisk indicates statistically different values (T-test, P< 0.05) between each group and the group fed sterile blood. CAT (D-G) Tissue-specific silencing in females fed with 5.4 × 107 CFU/mL E. coli HT115(DE3) expressing dsRNA CAT or blood alone. (D) Anterior midgut, (E) posterior midgut, (F) fat body and (G) ovaries from each individual were processed to measure expression of CAT, relative to controls. Bars represent SEM, three biological replicates (n = 3 per replicate). In all, asterisk indicates statistically different values as compared to controls fed with blood alone (T-test, P< 0.05).
For CAT knockdown experiments, females were fed on the highest dose of E. coli as established with the RHBP construct. The knockdown response to bacteria expressing dsRNA for CAT was observed at least at one time point for all tissues tested (Fig. 1D-G). Gene expression levels were significantly reduced, relative to the controls, on day one in the anterior midgut (69%), days three and five in the posterior midgut (82 and 96% respectively), days three and five in the fat body (65 and 76% respectively), and days one and five in the ovary (94 and 96% respectively). CAT knockdown varied between 65–96% in all tissues and time points tested but showed no significant difference between tissues (ANOVA, p>0.05).
Establishment of feeding conditions and verification of knockdown in nymphs
Optimal concentrations of E. coli for feeding first and third instar nymphs were determined using bacteria producing RHBP or CAT dsRNA. Third instars tolerated the same concentrations of bacteria as the adults without compromising viability (Table S1 in S1 Text). First instars showed 0–8% mortality in the control groups with a concentration of 2.5 × 107 CFU/mL. First instars fed blood containing bacteria expressing RHBP dsRNA showed 30% mortality rates (Table S1 in S1 Text). The expression of RHBP and CAT in third instar nymphs was reduced by 99% on days three, five and eight after feeding (Fig. 2B and 2C). Molting was completely inhibited in surviving nymphs fed bacteria expressing RHBP or CAT dsRNA, as compared to nymphs fed blood alone (Table S1 in S1 Text). Only 20% of surviving third instars fed bacteria expressing RHBP or CAT dsRNA molted to the fourth instar, compared to 100% in the control groups (Fig. 2A). The results show that E. coli producing dsRNA for RHBP or CAT affect immature stage development and produce RHBP or CAT knockdown in third instars.
Figure 2. Inhibition of molting and reduction in transcription levels of RHBP and CAT in third instar nymphs.
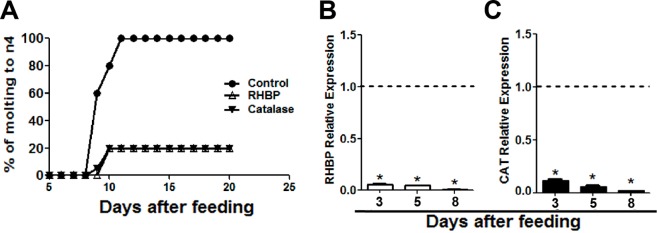
Nymphs were fed blood with E. coli producing RHBP and CAT dsRNA. (A) Reduction of molting in third instar nymphs fed bacteria producing RHBP or CAT dsRNA as compared with nymphs fed blood without bacteria and with bacteria expressing ANT dsRNA (two biological replicates, n = 10 each). (B) Relative expression of RHBP in third instar nymphs fed bacteria producing RHBP dsRNA (two biological replicates, n = 3 each). (C) Relative expression of CAT in midguts of third instar nymphs fed with bacteria producing RHBP dsRNA (two biological replicates, n = 3 each). Asterisk indicates statistically different values compared with the control fed blood alone (T-test, P< 0.05).
Physiological effects of RHBP and CAT silencing in adult females
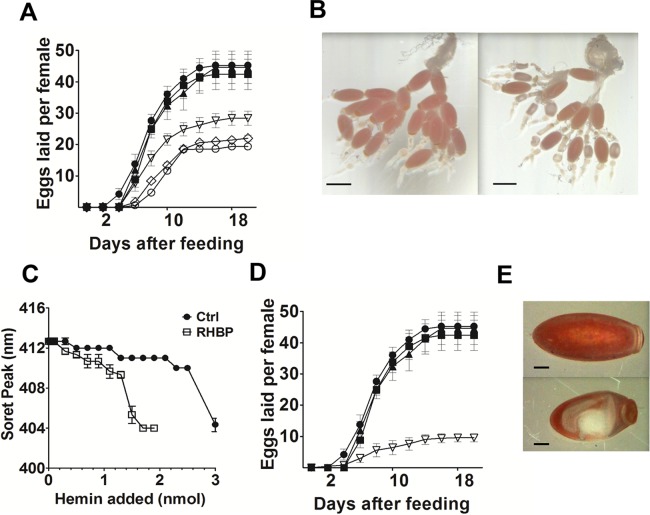
Vitellogenesis in the telotrophic ovary begins within two days after the blood meal. After feeding, normal individuals initiate the first of three rounds of terminal oocyte development, consisting of approximately 14 eggs per round, for a total of approximately 42 eggs per 21 day oviposition cycle [38]. Each round produces terminal oocytes termed T0, T1 and T2. Females fed E. coli induced to express RHBP dsRNA showed a delay of one week in the first oviposition event, leading to a 43% reduction in the total number of eggs produced as compared to controls (Fig. 3A). During the oviposition cycle, the number of eggs oviposited by individuals of all groups was monitored individually and no significant difference was observed between the control groups (ANOVA, Tukey post hoc test, p>0.05). In the ovary of RHBP knockdown animals, the terminal oocyte development (T0) was inhibited at day ten post feeding (Fig. 3B). At the highest bacterial concentration, 5.54 × 107 CFU/mL blood, the total reduction of eggs was 43%. Of the oviposited eggs, 16% (15 of 96) were white and non-viable, suggesting a reduction of heme-RHBP endocytosis in non-viable eggs [26]. The hemolymph protein profile was analyzed by titration of apo-RHBP at days three and seven after feeding. Comparing with the control groups fed blood alone or with E. coli expressing ANT dsRNA, a significant reduction of circulating apo-RHBP was observed in females fed E. coli expressing RHBP dsRNA at the seventh day post ingestion (T-test, P< 0.05) (Fig. 3C).
Figure 3. Physiological effects in adult females fed with E. coli expressing RHBP or CAT dsRNA.
Reduction in oviposition, egg development and circulating apo-RHBP in females fed with E. coli producing RHBP dsRNA. (A) 20 day oviposition cycle. In black the three control groups (blood alone (circle), bacteria without dsRNA (square) and bacteria with ANT dsRNA (triangle)), in white the groups fed bacteria expressing RHBP dsRNA at three different concentrations 3.35 × 107 CFU/mL blood (triangle), 4.02 × 107 CFU/mL blood (rhombus) and 5.4 × 107 CFU/ml blood (circle) (n = 5). Error bars represent SEM of three biological replicates. For one of the replicates, an additional control of uninduced RHBP bacteria was used, the insects (n = 8) showed no visible reduction from the normal number of eggs, ranging between 38–50 eggs/female. (B) Effects of the RHBP dsRNA in the ovaries of adult females of R. prolixus 10 days after feeding: left panel shows a control R. prolixus ovary. Right panel shows the effect of the inhibition of RHBP by feeding blood with bacteria expressing RHBP dsRNA. Bar = 1mm. (C) Reduction of apo-RHBP present in hemolymph seven days after feeding blood alone (ctrl) or 5.4 × 107 CFU/mL blood bacteria expressing RHBP dsRNA (RHBP). Titration with hemin showed a significant reduction of the circulating apo-RHBP in knocked down insects (T-test, P = 0.0088). Bars represent SEM, three biological replicates, 1 representative female/replicate. As additional control of sample integrity, SDS-PAGE was performed to corroborate the protein profile. (D) Oviposition cycles in females fed blood alone (black circle), 5.4 × 107 CFU/mL blood bacteria without dsRNA (black square), bacteria expressing ANT dsRNA (black triangle), and bacteria expressing CAT dsRNA (white triangle), (two biological replicates, n = 6 each). (E) The dehydration phenotype was observed in 20% of the eggs laid by females fed with bacteria expressing CAT dsRNA, at 5.4 × 107 CFU/ml of blood. Bar = 0.2 mm.
To evaluate if silencing had long term effects, a group of females was fed the bacteria during two consecutive gonotrophic cycles and the ovipositon was observed in both cycles. No difference in oviposition was observed between the first and the second feedings for the RHBP treatment (T-test, P>0.05) (S3 Fig.). There was no significant difference among the control groups (ANOVA, p>0.05).
When oviposition and egg development were evaluated in females fed E. coli expressing CAT dsRNA, the total number was reduced to 7–14 eggs per female, when normal females lay 39–54 eggs (Fig. 3D). Among a total of 9±3 eggs oviposited per female, 2±1 eggs (20%) presented a dehydrated phenotype (Fig. 3E) and were non-viable. To verify that the nonviability of the eggs was not related to lack of fertilization of the females, we performed a PCR for the Y chromosome using DNA extracted from normal eggs from control females and from dehydrated eggs from females knocked-down for CAT (S4 Fig.). All the samples were fertilized. ROS levels were observed and CAT specific activity was measured in the anterior midgut of females at day six after feeding the bacteria. The results showed a visible increase in midgut ROS levels upon CAT knockdown (Fig. 4A), together with a 76.89% (±16.79) reduction in CAT specific activity in relation to controls (Mann-Whitney, P<0.05) (Fig. 4B).
Figure 4. Reactive oxygen species and CAT specific activity in midguts of females fed with E. coli HT115(DE3) expressing CAT dsRNA.
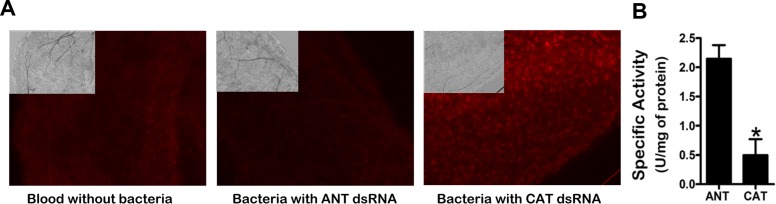
Females were fed blood alone, with E. coli HT115(DE3) expressing ANT dsRNA or CAT dsRNA, at 5.54 × 107 CFU/mL blood. Midguts were dissected six days after feeding, incubated with dihydroethidium (DHE) and photographed under epifluorescence microscopy (Zeiss Observer.Z1 with Zeiss Axio Cam MrM using a filter set 10 (Exc 450–490 nm/ emission 515–565 nm)). Photographs show representative individuals from each group, inserts are differential interference contrast images. (B) Mean specific activity of CAT in insects fed E. coli HT115(DE3) expressing dsRNA CAT. Two biological replicates, n = 3 each. Error bars represent standard error of the mean. Asterisk indicates significant difference from control (T-test, P< 0.05).
RNAi effects in adult females after feeding with R. rhodnii expressing RHBP dsRNA hairpins
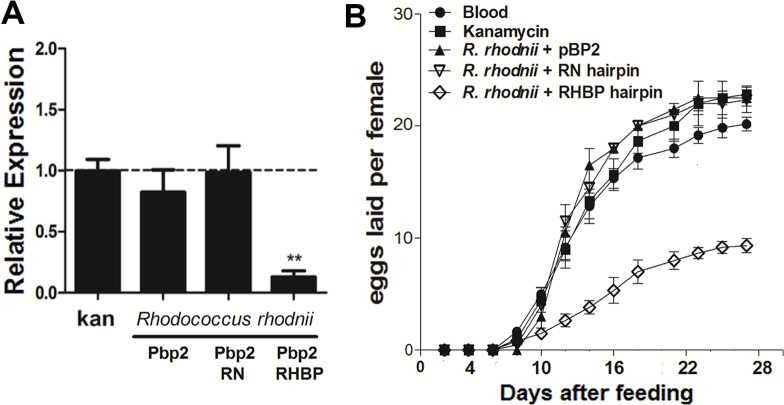
We amplified, cloned, sequenced, and expressed a RHBP dsRNA hairpin in the natural symbiont of R. prolixus, R. rhodnii, using pBP2lacZ, an integration plasmid with a constitutive expression promoter and a kanamycin resistance gene (SI Materials and Methods). A random nucleotide (RN) hairpin was used as a control. Insects were fed blood containing kanamycin and R. rhodnii that expressed these hairpins, bacteria with integrated plasmid without inserts, or blood free of bacteria. We used a concentration of bacteria similar to the normal level in the R. prolixus midgut after starvation (105CFU). Inhibition of RHBP mRNA expression was confirmed by q-PCR (Fig. 5A), observing a significant (T test, P<0.05) reduction (87%) of the expression five days after the feeding. Oviposition was affected in a similar manner as with E. coli, obtaining a significant reduction (47%) of the total number of eggs laid (ANOVA with Bonferroni post hoc test, P<0.001). No white eggs where observed and the viability of the laid eggs was 100%.
Figure 5. Reduction in RHBP expression and oviposition in females fed with R. rhodnii producing a RHBP dsRNA hairpin.
(A) Females were fed blood with kanamycin alone (kan) or containing 105 CFU/mL R. rhodnii transformed with pBP2lac without insert (pBP2lac), expressing a RHBP dsRNA hairpin (pBP2lacRHBP) or a random nucleotide hairpin (pBP2lacRN). Error bars represent SEM of three biological replicates (n = 12 each). Asterisk indicates statistically different values (T-test, P< 0.05). (B) Reduction of eggs in one oviposition cycle. Three biological replicates (n = 6 each). Error bars represent SEM. Asterisks denote statistically significant values relative to control fed blood alone (T test, P<0.05).
Discussion
Oral administration of E. coli expressing dsRNA for two R. prolixus genes involved in oxidative stress achieved systemic gene knockdown in adult and immature stages, with subsequent effects on oviposition and molting. Knockdown was effective in females and third instars, showing effects on expression profiles at least one week after oral delivery of bacteria. Lower concentrations of the natural symbiont of the insect, R. rhodnii, achieved similar effects. With both bacterial systems, we show deleterious effects on insect fitness. We propose that the E. coli system may be used as an effective high throughput system to screen for targets to be further tested with the symbiont, R. rhodnii.
Insects fed E. coli expressing RHBP dsRNA recovered to control expression levels ten days after feeding. This recovery is also evident in females fed bacteria during two consecutive oviposition cycles. The oviposition phenotype during the second knockdown event is not affected by the previous knockdown. The oviposition phenotype with R. rhodnii suggests that RHBP must have reached normal levels after an initial knockdown. Oral delivery of bacteria expressing dsRNA produces a temporary knockdown that is shorter-lived compared with knockdown generated by intrathoracic injection of one microgram of RHBP dsRNA [30]. Temporary knockdown is also seen after feeding nymphs 1 μg/μL of dsRNA for salivary gland nitrophorins [39]. Although the temporary effects may be construed as a limitation of our system, the estimated dsRNA dose with E. coli is 100 times lower compared to oral delivery of pure dsRNA and similar to the intrathoracic inoculation doses, suggesting that bacteria are effective dsRNA vehicles through the oral route. If the target is properly selected, the temporary silencing should produce strong enough phenotypes to affect vector development and/or competence.
Lower symbiont concentrations were needed to induce knockdown compared to E. coli. The E. coli strain is induced before oral delivery to expresses dsRNA from high copy number plasmids whereas R. rhodnii contains a hairpin construct under a constitutive promoter that is integrated into the chromosome. The dsRNA expression kinetics of the symbiont in the midgut remains to be determined. Symbionts are known to replicate in the midgut and are thought to subsequently lyse [23]. Whether dsRNA was released upon bacterial lysis is not known. The pathway by which the dsRNA is taken up from the intestine and delivered to other tissues is not fully understood either. Some insects have transmembrane proteins thought to transport dsRNA [40, 41]. Alternatively, there may be an endocytic pathway [42]. Regardless of the dsRNA release mechanism and uptake route, the silencing mechanism with both E. coli and the symbiont had strong physiological effects. Silencing efficacy may be improved by optimizing the symbiont expression system, taking into consideration the dynamics of target gene expression and bacterial promoter activity in the insect midgut.
Catalase expression was reduced in various tissues, suggesting that silencing efficacy is not determined by the tissue proximity to the dsRNA source. Also, it appeared that the earlier the developing stage receives the bacterial treatment, the higher the impact it has on fitness, as first instars presented a 30% mortality rate. Taken together, the data suggest that CAT could be combined with other molecular targets to reduce insect fecundity and development through RNAi. The importance of CAT in parasite survival and insect fecundity has been demonstrated in other vector insects such as Anopheles gambiae [43] and Lutzomyia longipalpis [28]. Knockdown of CAT in An. gambiae resulted in a decrease in Plasmodium berghei burden [44] and a decrease of 45% larvae produced by female mosquitoes [43], proving that ROS detoxification by CAT is involved in immunity and protects the ovary and embryo from oxidative damage. In L. longipalpis CAT knockdown reduces Leishmania mexicana burden [45] as well as female fitness and reproductive capacity [28]. Future studies should determine the effect of silencing CAT on T. cruzi development. If CAT silencing affects both, parasite and vector development, this strategy may function to simultaneously reduce transmission and vector colonization.
Given that our proof-of-concept with E. coli was efficient at gene knockdown, we propose to use E. coli to develop a high throughput screen for novel targets for this strategy and as a tool to study vector physiology. The E. coli strain provides a better screening tool because it can be transformed at high efficiencies with extrachromosomal high copy plasmids and it grows more quickly. In contrast, the R. rhodnii transformation system requires random integration into one of several potential chromosomal attachment sites. This makes it more difficult to select for transformants that have the same genetic background for screening purposes. Molecules identified through E. coli screening can be selected for integration into specific attachment sites in the R. rhodnii chromosome for further experiments. The E. coli system may be used in future studies as a research tool to identify target genes that produce higher nymph or egg mortality levels. It may also be used to evaluate the effect of silencing triatomine genes on T. cruzi interactions with the vector and potential transmission-blocking effects that may complement the control strategy.
Selection of potential molecular targets should consider the kinetics of gene expression after feeding and the half-life of the protein being silenced. An ideal target in the development of a symbiont-based control strategy with R. rhodnii should be expressed maximally one week after the blood meal to take advantage of the bacterial replication cycle in the midgut [23]. In addition, the protein being knocked down should have a high turnover rate, as the silencing effect may be overshadowed by the presence of previously produced protein. The availability of the R. prolixus genome and tissue-specific cDNA sequences will greatly facilitate target screening for this strategy.
We showed for the first time that transformed symbionts fed in blood are effective at delivering dsRNA and silencing genes in a hemipteran disease vector. Future studies will evaluate silencing efficacy after delivery in a paste-like formulation to simulate natural coprophagous transmission [16]. The bacterial integration system we used is stable even in the absence of antibiotic selection once introduced into the vector [46]. The availability of effective systems for symbiont delivery through simulated feces and stable transformation make it feasible to produce gene-silencing microbiota. Horizontal transmission among exposed insects can be a sustainable alternative to traditional insecticides. This could reduce vector competence and development, with low ecological impact due to target specificity [10]. The selection of resistance in natural populations may be minimized if multiple genes are simultaneously targeted, similar to what occurs with Bacillus thuringiensis in mosquitoes, where Cry and Cyt toxins synergize with each other and avoid resistance [47]. The production of bacteria that express dsRNA for several molecular targets simultaneously and constitutively would be recommended [48]. The future applications of the genetic modification of insect microbiota to produce RNAi are wide. These post-genomic tools for the study of insect physiology, vector-parasite interactions and ultimately control strategies may be applied to other insects of importance to human and animal health.
Supporting Information
S1 Table in S1 Text. Mortality and molting rates of first and third instar nymphs of R. prolixus fed with E. coli expressing dsRNA for RHBP or CAT.
(PDF)
(TIF)
(TIF)
(TIF)
(TIF)
Acknowledgments
We wish to express our gratitude to A. B. Walter-Nuno for her expert assistance in RNAi experiments; José de Souza Lima Jr. for maintaining our colony of Rhodnius prolixus at UFRJ, JG Juarez and H Perdomo at UVG, S. J. Tadeu, and S. R. de Cassia for valuable technical assistance.
Data Availability
A protected Excel file Taracena_et_al_2014_Real Time_Database is available in the www.acervosalud.net menu under "Reportes". You can access it directly at: http://www.acervosalud.net/index.php?option=com_content&view=article&id=77&Itemid=156&lang=es. Further information regarding the database can be obtained by contacting Celia Cordón-Rosales, Director of the Center for Health Studies at Chagas@ces.uvg.edu.gt
Funding Statement
The work was made possible by funding to PMP from Fondo Nacional de Ciencia y Tecnología, -FONACYT- of the National Secretariat for Science and Technology - SENACYT- and the support of the National Council for Science and Technology -CONCYT- of Guatemala. CL received funding from the Latin American and Caribbean Research Exchange Grant to support CU. This work was also supported by grants from the Conselho Nacional de Desenvolvimento Científico e Tecnológico, the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior, the Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro, the Programa de Apoio a Núcleos de Excelência, and the Howard Hughes Medical Institute. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Zhou X, Wheeler MM, Oi FM, Scharf ME (2008) RNA interference in the termite Reticulitermes flavipes through ingestion of double-stranded RNA. Insect Biochem Mol Biol 38: 805–815. 10.1016/j.ibmb.2008.05.005 [DOI] [PubMed] [Google Scholar]
- 2. Whyard S, Singh AD, Wong S (2009) Ingested double-stranded RNAs can act as species-specific insecticides. Insect Biochem Mol Biol 39: 824–832. 10.1016/j.ibmb.2009.09.007 [DOI] [PubMed] [Google Scholar]
- 3. Nunes FM, Simoes ZL (2009) A non-invasive method for silencing gene transcription in honeybees maintained under natural conditions. Insect Biochem Mol Biol 39: 157–160. 10.1016/j.ibmb.2008.10.011 [DOI] [PubMed] [Google Scholar]
- 4. Ghanim M, Kontsedalov S, Czosnek H (2007) Tissue-specific gene silencing by RNA interference in the whitefly Bemisia tabaci (Gennadius). Insect Biochem Mol Biol 37: 732–738. 10.1016/j.ibmb.2007.04.006 [DOI] [PubMed] [Google Scholar]
- 5. Walshe DP, Lehane SM, Lehane MJ, Haines LR (2009) Prolonged gene knockdown in the tsetse fly Glossina by feeding double stranded RNA. Insect Mol Biol 18: 11–19. 10.1111/j.1365-2583.2008.00839.x [DOI] [PubMed] [Google Scholar]
- 6. Soares CA, Lima CM, Dolan MC, Piesman J, Beard CB, et al. (2005) Capillary feeding of specific dsRNA induces silencing of the isac gene in nymphal Ixodes scapularis ticks. Insect Mol Biol 14: 443–452. 10.1111/j.1365-2583.2005.00575.x [DOI] [PubMed] [Google Scholar]
- 7. Yu N, Christiaens O, Liu J, Niu J, Cappelle K, et al. (2013) Delivery of dsRNA for RNAi in insects: an overview and future directions. Insect Sci 20: 4–14. 10.1111/j.1744-7917.2012.01534.x [DOI] [PubMed] [Google Scholar]
- 8. Baum JA, Bogaert T, Clinton W, Heck GR, Feldmann P, et al. (2007) Control of coleopteran insect pests through RNA interference. Nat biotechnol 25: 1322–1326. 10.1038/nbt1359 [DOI] [PubMed] [Google Scholar]
- 9. Tian H, Peng H, Yao Q, Chen H, Xie Q, et al. (2009) Developmental control of a lepidopteran pest Spodoptera exigua by ingestion of bacteria expressing dsRNA of a non-midgut gene. PLoS One 4: e6225 10.1371/journal.pone.0006225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Singh AD, Wong S, Ryan CP, Whyard S (2013) Oral delivery of double-stranded RNA in larvae of the yellow fever mosquito, Aedes aegypti: implications for pest mosquito control. J Insect Sci 13: 69 10.1673/031.013.6901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zhang X, Zhang J, Zhu KY (2010) Chitosan/double-stranded RNA nanoparticle-mediated RNA interference to silence chitin synthase genes through larval feeding in the African malaria mosquito (Anopheles gambiae). Insect Mol Biol 19: 683–693. 10.1111/j.1365-2583.2010.01029.x [DOI] [PubMed] [Google Scholar]
- 12. Hashimoto K, Schofield CJ (2012) Elimination of Rhodnius prolixus in Central America. Parasit Vectors 5: 45 10.1186/1756-3305-5-45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Vassena CV, Picollo MI, Zerba EN (2000) Insecticide resistance in Brazilian Triatoma infestans and Venezuelan Rhodnius prolixus . Med Vet Entomol 14: 51–55. 10.1046/j.1365-2915.2000.00203.x [DOI] [PubMed] [Google Scholar]
- 14. Schofield CJ, Jannin J, Salvatella R (2006) The future of Chagas disease control. Trends Parasitol 22: 583–588. 10.1016/j.pt.2006.09.011 [DOI] [PubMed] [Google Scholar]
- 15. Picollo MI, Vassena C, Santo Orihuela P, Barrios S, Zaidemberg M, et al. (2005) High resistance to pyrethroid insecticides associated with ineffective field treatments in Triatoma infestans (Hemiptera: Reduviidae) from Northern Argentina. J Med Entomol 42: 637–642. 10.1603/0022-2585(2005)042%5B0637:HRTPIA%5D2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- 16. Durvasula RV, Gumbs A, Panackal A, Kruglov O, Aksoy S, et al. (1997) Prevention of insect-borne disease: an approach using transgenic symbiotic bacteria. Proc Natl Acad Sci USA 94: 3274–3278. 10.1073/pnas.94.7.3274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wang S, Ghosh AK, Bongio N, Stebbings KA, Lampe DJ, et al. (2012) Fighting malaria with engineered symbiotic bacteria from vector mosquitoes. Proc Natl Acad Sci USA 109: 12734–12739. 10.1073/pnas.1204158109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Weiss B, Aksoy S (2011) Microbiome influences on insect host vector competence. Trends Parasitol 27: 514–522. 10.1016/j.pt.2011.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Beard CB, O'Neill SL, Tesh RB, Richards FF, Aksoy S (1992) Transformation of an insect symbiont and expression of a foreign gene in the Chagas' disease vector Rhodnius prolixus . Am J Trop Med Hyg 46: 195–200. [DOI] [PubMed] [Google Scholar]
- 20. Beard CB, Dotson EM, Pennington PM, Eichler S, Cordon-Rosales C, et al. (2001) Bacterial symbiosis and paratransgenic control of vector-borne Chagas disease. Int J Parasitol 31: 621–627. 10.1016/S0020-7519(01)00165-5 [DOI] [PubMed] [Google Scholar]
- 21. Wigglesworth VB (1936) Symbiotic bacteria in a blood-sucking insect, Rhodnius prolixus Stahl (Hemiptera, Triatomidae). Parasitol 28: 284–289. 10.1017/S0031182000022459 [DOI] [Google Scholar]
- 22. Brecher G, Wigglesworth VB (1944) The transmission of Actinomyces rhondii Erikson in Rhodnius prolixus Stal (Hemiptera) and its influence on the growth of the host. Parasitol 35: 220–224. 10.1017/S0031182000021648 [DOI] [Google Scholar]
- 23. Eichler S, Schaub GA (2002) Development of symbionts in triatomine bugs and the effects of infections with trypanosomatids. Exp Parasitol 100: 17–27. 10.1006/expr.2001.4653 [DOI] [PubMed] [Google Scholar]
- 24. Dasgupta S, Fernandez L, Kameyama L, Inada T, Nakamura Y, et al. (1998) Genetic uncoupling of the dsRNA-binding and RNA cleavage activities of the Escherichia coli endoribonuclease RNase III--the effect of dsRNA binding on gene expression. Mol Microbiol 28: 629–640. 10.1046/j.1365-2958.1998.00828.x [DOI] [PubMed] [Google Scholar]
- 25. Dansa-Petretski M, Ribeiro JMC, Atella GC, Masuda H, Oliveira PL (1995) Antioxidant Role of Rhodnius prolixus Heme-binding Protein. J Biol Chem 270: 10893–10896. 10.1074/jbc.270.18.10893 [DOI] [PubMed] [Google Scholar]
- 26. Braz GR, Moreira MF, Masuda H, Oliveira PL (2002) Rhodnius heme-binding protein (RHBP) is a heme source for embryonic development in the blood-sucking bug Rhodnius prolixus (Hemiptera, Reduviidae). Insect Biochem Mol Biol 32: 361–367. 10.1016/S0965-1748(01)00163-1 [DOI] [PubMed] [Google Scholar]
- 27. Paes MC, Oliveira MB, Oliveira PL (2001) Hydrogen peroxide detoxification in the midgut of the blood-sucking insect, Rhodnius prolixus . Arch Insect Biochem Physiol 48: 63–71. 10.1002/arch.1058 [DOI] [PubMed] [Google Scholar]
- 28. Diaz-Albiter H, Mitford R, Genta FA, Sant'Anna MR, Dillon RJ (2011) Reactive oxygen species scavenging by catalase is important for female Lutzomyia longipalpis fecundity and mortality. PLoS One 6: e17486 10.1371/journal.pone.0017486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Tappel AL (1955) Unsaturated lipid oxidation catalyzed by hematin compounds. The J Biol Chem 217: 721–733. [PubMed] [Google Scholar]
- 30. Walter-Nuno AB, Oliveira MP, Oliveira MF, Goncalves RL, Ramos IB, et al. (2013) Silencing of maternal heme-binding protein causes embryonic mitochondrial dysfunction and impairs embryogenesis in the blood sucking insect Rhodnius prolixus . J Biol Chem 288: 29323–29332. 10.1074/jbc.M113.504985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Oliveira PL, Kawooya JK, Ribeiro JM, Meyer T, Poorman R, et al. (1995) A heme-binding protein from hemolymph and oocytes of the blood-sucking insect, Rhodnius prolixus . Isolation and characterization. J Biol Chem 270: 10897–10901. [DOI] [PubMed] [Google Scholar]
- 32. Machado EA, Oliveira PL, Moreira MF, de Souza W, Masuda H (1998) Uptake of Rhodnius heme-binding protein (RHBP) by the ovary of Rhodnius prolixus . Arch Insect Biochem Physiol 39: 133–143. 10.1002/(SICI)1520-6327(1998)39:4%3C133::AID-ARCH1%3E3.3.CO;2-4 [DOI] [PubMed] [Google Scholar]
- 33. Paiva-Silva GO, Sorgine MH, Benedetti CE, Meneghini R, Almeida IC, et al. (2002) On the biosynthesis of Rhodnius prolixus heme-binding protein. Insect Biochem Mol Biol 32: 1533–1541. 10.1016/S0965-1748(02)00074-7 [DOI] [PubMed] [Google Scholar]
- 34. Chance B, Sies H, Boveris A (1979) Hydroperoxide metabolism in mammalian organs. Physiol Rev 59: 527–605. [DOI] [PubMed] [Google Scholar]
- 35. Graca-Souza AV, Maya-Monteiro C, Paiva-Silva GO, Braz GR, Paes MC, et al. (2006) Adaptations against heme toxicity in blood-feeding arthropods. Insect Biochem Mol Biol 36: 322–335. 10.1016/j.ibmb.2006.01.009 [DOI] [PubMed] [Google Scholar]
- 36. Timmons L, Tabara H, Mello CC, Fire AZ (2003) Inducible systemic RNA silencing in Caenorhabditis elegans . Mol Biol Cell 14: 2972–2983. 10.1091/mbc.E03-01-0858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Cramptom J (1997) Molecular biology of insect disease vectors: a methods manual; Beard C, Louis C, editors: Chapman & Hall: 578 p. [Google Scholar]
- 38. Davey KG (1987) Inputs to the hormonal control of egg development in Rhodnius prolixus . Mem Inst Oswaldo Cruz 82 Suppl 3: 103–108. 10.1590/S0074-02761987000700020 [DOI] [PubMed] [Google Scholar]
- 39. Araujo RN, Santos A, Pinto FS, Gontijo NF, Lehane MJ, et al. (2006) RNA interference of the salivary gland nitrophorin 2 in the triatomine bug Rhodnius prolixus (Hemiptera: Reduviidae) by dsRNA ingestion or injection. Insect Biochem Mol Biol 36: 683–693. 10.1016/j.ibmb.2006.05.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Xu W, Han Z (2008) Cloning and phylogenetic analysis of sid-1-like genes from aphids. J Insect Sci 8: 1–6. 10.1673/031.008.3001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Aronstein K, Pankiw T, Saldivar E (2006) Sid-1 is implicated in systemic gene silencing in the honey bee. J Apicultural Res 45: 20–24. [Google Scholar]
- 42. Ulvila J, Parikka M, Kleino A, Sormunen R, Ezekowitz RA, et al. (2006) Double-stranded RNA is internalized by scavenger receptor-mediated endocytosis in Drosophila S2 cells. J Biol Chem 281: 14370–14375. 10.1074/jbc.M513868200 [DOI] [PubMed] [Google Scholar]
- 43. DeJong RJ, Miller LM, Molina-Cruz A, Gupta L, Kumar S, et al. (2007) Reactive oxygen species detoxification by catalase is a major determinant of fecundity in the mosquito Anopheles gambiae . Proc Natl Acad Sci USA 104: 2121–2126. 10.1073/pnas.0608407104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Molina-Cruz A, DeJong RJ, Charles B, Gupta L, Kumar S, et al. (2008) Reactive oxygen species modulate Anopheles gambiae immunity against bacteria and Plasmodium. J Biol Chem 283: 3217–3223. 10.1074/jbc.M705873200 [DOI] [PubMed] [Google Scholar]
- 45. Diaz-Albiter H, Sant'Anna MR, Genta FA, Dillon RJ (2012) Reactive oxygen species-mediated immunity against Leishmania mexicana and Serratia marcescens in the sand phlebotomine fly Lutzomyia longipalpis . J Biol Chem 287: 23995–24003. 10.1074/jbc.M112.376095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Dotson EM, Plikaytis B, Shinnick TM, Durvasula RV, Beard CB (2003) Transformation of Rhodococcus rhodnii, a symbiont of the Chagas disease vector Rhodnius prolixus, with integrative elements of the L1 mycobacteriophage. Infect Genet Evol 3: 103–109. 10.1016/S1567-1348(03)00002-9 [DOI] [PubMed] [Google Scholar]
- 47. Gómez I, Pardo-López L, Muñoz-Garay C, Fernandez LE, Pérez C, et al. (2007) Role of receptor interaction in the mode of action of insecticidal Cry and Cyt toxins produced by Bacillus thuringiensis . Peptides 28: 169–173. 10.1016/j.peptides.2006.06.013 [DOI] [PubMed] [Google Scholar]
- 48. Brevault T, Heuberger S, Zhang M, Ellers-Kirk C, Ni X, et al. (2013) Potential shortfall of pyramided transgenic cotton for insect resistance management. Proc Natl Acad Sci USA 110: 5806–5811. 10.1073/pnas.1216719110 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
S1 Table in S1 Text. Mortality and molting rates of first and third instar nymphs of R. prolixus fed with E. coli expressing dsRNA for RHBP or CAT.
(PDF)
(TIF)
(TIF)
(TIF)
(TIF)
Data Availability Statement
A protected Excel file Taracena_et_al_2014_Real Time_Database is available in the www.acervosalud.net menu under "Reportes". You can access it directly at: http://www.acervosalud.net/index.php?option=com_content&view=article&id=77&Itemid=156&lang=es. Further information regarding the database can be obtained by contacting Celia Cordón-Rosales, Director of the Center for Health Studies at Chagas@ces.uvg.edu.gt