Abstract
Rationale
Although striatal dopamine (DA) is important in alcohol abuse, the nature of DA release during actual alcohol drinking is unclear, since drinking includes self-administration of both conditioned flavor stimuli (CS) of the alcoholic beverage and subsequent intoxication, the unconditioned stimulus (US).
Objectives
Here we used a novel self-administration analog to distinguish nucleus accumbens (NAcc) DA responses specific to the CS and US.
Methods
Right-handed male heavy drinkers (n=26) received 3 positron emission tomography (PET) scans with the D2/D3 radioligand [11C]raclopride (RAC), and performed a pseudo self-administration task that separately administered a flavor CS of either a habitually consumed beer or the appetitive control Gatorade®, concomitant with the US of ethanol intoxication (0.06 g/dL IV administration) or IV saline. Scan conditions were Gatorade flavor + saline (Gat&Sal); Gatorade flavor + ethanol (Gat&Eth); and beer flavor + ethanol (Beer&Eth).
Results
Ethanol (US) reduced RAC binding (inferring DA release) in the left (L) NAcc [Gat&Sal > Gat&Eth]. Beer flavor (CS) increased DA in the right (R) NAcc [Gat&Eth > Beer&Eth]. The combination of beer flavor and ethanol (CS + US), [Gat&Sal > Beer&Eth], induced DA release in bilateral NAcc. Self-reported intoxication during scanning correlated with L NAcc DA release. Relative to saline, infusion of ethanol increased alcoholic drink wanting.
Conclusions
Our findings suggest lateralized DA function in the NAcc, with L NAcc DA release most reflecting intoxication, R NAcc DA release most reflecting the flavor CS, and the conjoint CS+US producing a bilateral NAcc response.
Keywords: ventral striatum, mesolimbic, alcoholism, conditioned response, cue reactivity, reward, operant, alcohol abuse, instrumental conditioning, alcohol seeking
Introduction
Mesolimbic dopamine (DA) in the NAcc (within the ventral striatum; VST) has long been postulated to be central to reward (Robinson and Berridge, 1993, 2008; Schultz et al, 1997; Weiss et al, 1993). In animals, ethanol provokes NAcc DA release, (e.g. Di Chiara and Imperato, 1988), although NAcc DA transmission is also provoked by alcohol’s conditioned stimuli (CS), such as its olfactory and/or gustatory properties (Doyon et al, 2005). Drug-paired CS can exert powerful control over behavior, such as eliciting drug-seeking (for review see Crombag et al, 2008) or craving (e.g. Grant et al, 1996; Tiffany, 1999). We recently reported the first study on the effects of alcohol-related flavor CS on DA in humans. Using positron emission tomography (PET) with the DA D2/D3 ligand [11C]raclopride (RAC) we demonstrated that the flavor of a preferred beer (absent a measurable breath alcohol concentration) is sufficient to induce DA release in the right NAcc (Oberlin et al, 2013). Alcohol intoxication itself (the unconditioned stimulus; US) has been more widely studied with RAC-PET. In one study, intravenous (IV) alcohol infusion (devoid of alcohol’s usual aroma, flavor, and intra-oral sensations) induced bilateral (Ramchandani et al, 2011) DA release, although only in subjects carrying the minor allele of the OPRM1 mu-opioid receptor. Other studies found no effect with IV alcohol (Yoder et al, 2007; Yoder et al, 2005), or found it only when alcohol was unexpected (Yoder et al, 2009). However, two RAC-PET studies reported bilateral VST DA release when social drinkers consumed alcoholic beverages orally, thus combining alcohol’s intraoral somatosensory CS effects with the US of intoxication (Boileau et al, 2003; Urban et al, 2010); a similar study of oral consumption reported bilateral VST DA release, but only in high-risk subjects (Setiawan et al, 2014).
To more clearly ascribe DA responses to the elements of flavor and other intra-oral sensory properties (the CS) versus intoxication effects (the US), we separated alcohol’s pharmacologic actions (administered intravenously) from its normally conditioned sensory properties (delivered orally). We did so using a paradigm that preserves some instrumental aspect of normal human drinking (Sanchis-Segura and Spanagel, 2006 for review). Operant behavior may be crucial to VST’s DA signaling (Bjork and Hommer, 2007), as self-administration and approach behavior in animals increases NAcc DA (Phillips et al, 2003; Weiss et al, 1993). To that end, subjects in this study had PET scans using a DA D2/D3 ligand while self-administering flavors of beer or an appetitive flavor control, Gatorade®, during intravenous infusion of either alcohol or saline (which subjects believed was also self-administered). By manipulating flavor and intoxication independently, we assessed: 1) the US of alcohol intoxication; 2) the CS of beer flavor; and 3) the combined effect of beer flavor and intoxication (CS plus US) as in naturalistic drinking.
Compared to our previous findings (Oberlin et al, 2013) in which the CS (beer flavor) was administered during “extinction” (i.e., without ensuing intoxication), we hypothesized that the added context of ethanol self-administration would increase the NAcc DA response.
Methods
Subjects
All procedures were approved by the Indiana University Institutional Review Board, and all subjects signed informed consents prior to study procedures. The 28 subjects recruited from the community were right-handed, male beer drinkers in good self-reported physical and mental health who reported beer as being one of their two most-often consumed alcoholic beverages. Two subjects were excluded from analyses; one for equipment failure during the study, and the other for excessive motion. Exclusionary criteria included: positive urine screen for illicit drugs, reported current illicit drug use or past treatment for substance dependence, current treatment-seeking for substance use, self-reported dislike of Gatorade, or self-report of any symptoms, diagnoses, or treatment consistent with a current or past major DSM-IV Axis I psychiatric disorder (aside from alcohol dependence), any history of neurological disease of cerebral origin, head injury resulting in loss of consciousness for > 20 minutes, current use of psychotropic medications, Beck Depression Inventory scores of > 9, or past/current problems in circulatory, endocrine, gastrointestinal, urinary, musculo-skeletal, or blood-related disorders. Subjects underwent in-person interviews that included the Semi-Structured Assessment for the Genetics of Alcoholism (SSAGA: Bucholz et al, 1994), the Timeline Followback (90 day TLFB: Sobell et al, 1986) to quantify recent drinking, and the Alcohol Use Disorder Identification Test (AUDIT: Saunders et al, 1993). The family history module of the SSAGA was also used to determine familial alcoholism, and to avoid possible fetal alcohol effects by excluding subjects whose mothers had probable alcoholism. Recent drinking ranged from social to heavy (Table 1). Two of the 26 subjects met DSM-IV criteria for alcohol dependence as assessed by the SSAGA; however, these subjects did not differ from the rest of the group in drinks per drinking day (7.2 ± 2.8 and 7.2 ± 2.5, respectively, mean ± SD). Four subjects reported weekly marijuana use within the past year, although no use within the past two months (recent abstinence was verified by urine tests); all other subjects’ past marijuana use was lower. Regular habitual cigarette smoking was exclusionary, although two subjects reported smoking up to 1 cigarette or cigar per week. None of these subjects participated in our previous RAC-PET gustatory study (Oberlin et al, 2013).
Table 1.
Subject Characteristics.
| Mean ± (SD) | Range | n(%) | |
|---|---|---|---|
| Age | 23.1 (3.3) | 21–34 | |
| Caucasian1 | - | - | 25 (96%) |
| Education | 14.8 (1.4) | 12–20 | |
| Drinks per week2 | 23.0 (12.0) | 9–64 | |
| Drinks per drinking day2 | 7.2 (2.5) | 3–12 | |
| Heavy drinking days per week2,3 | 2.2 (1.0) | 1–4 | |
| Age of first intoxication | 16.4 (2.2) | 12–21 | |
| AUDIT4 | 13.8 (4.5) | 9–27 | |
Two subjects self-identified as Hispanic/Latino.
From the Timeline Followback Interview.
Greater than 4 drinks per day.
Alcohol Use Disorder Identification Test.
Procedure
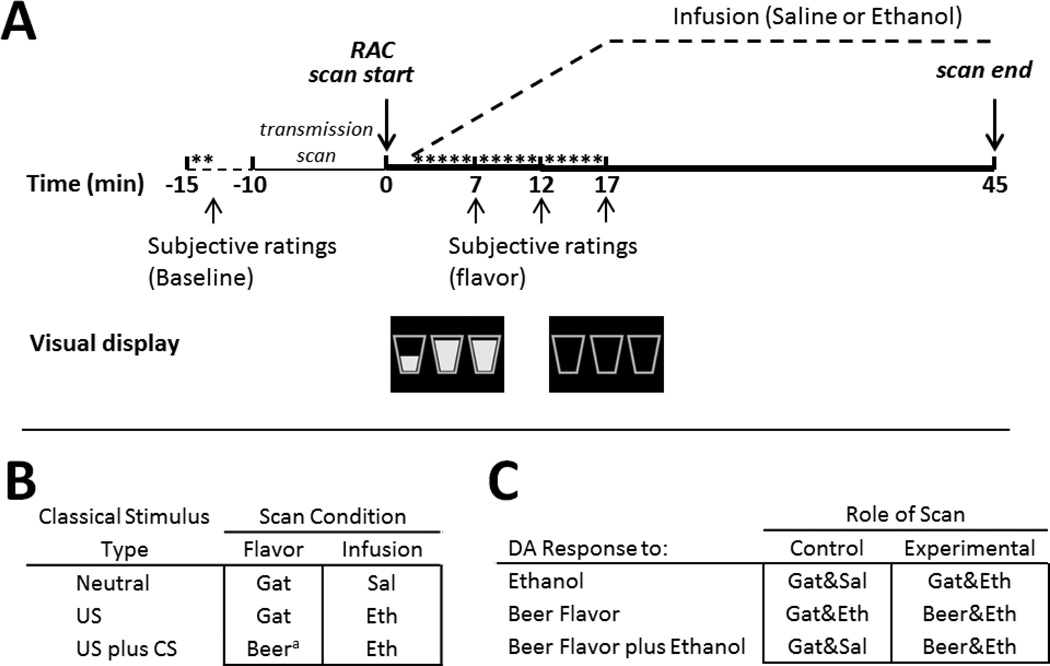
Subjects were told that they would be responding for flavor (beer or Gatorade) sprays and IV infusion (ethanol or saline) by pressing a mouse button, and that the flavor/infusion combination might vary across scans. Subjects underwent three RAC PET scans on two consecutive days (with an overnight stay at the Clinical Research Center): one scan on the first day and two scans at least two hours (6 half-lives) apart on the second day, with all three scans occurring at fixed times to minimize circadian variability (day 1/scan 1, 1:00pm; day 2/scan 2, 1:00pm; day 2/scan 3, 3:00pm). Scans involving ethanol infusion were always first and last, and were counterbalanced in order for presentation of beer and Gatorade flavor (beer first, n=12). Two flavors of Gatorade tastant (blue Glacier Freeze™ and Orange) were randomized across subjects. Subjects completed three scans while presented with Gatorade and saline (Gat&Sal), Gatorade and ethanol (Gat&Eth), and beer and ethanol (Beer&Eth), as illustrated in Figure 1B, with the two flavors of Gatorade employed so as to avoid one flavor becoming associated with ethanol intoxication. The beer used was each individual subject’s most frequently consumed brand.
Fig. 1.
(A) Single scan: stimuli and infusion. Baseline ratings followed two water sprays. For illustration, the drink images are shown here after 3 sips and 15 sips (empty). Time is relative to RAC injection. *Self-administered sprays of water or flavor. Dashed line illustrates ascending and then clamped breath alcohol concentration. For saline, the same pump rates were used as those needed to achieve the same alcohol time course. (B) Three-scan study design. aPreferred beer. US = unconditioned stimulus, CS = conditioned stimulus. (C) Relevant comparisons. Gat = Gatorade, Sal = saline, Eth = ethanol infusion targeted to breath alcohol concentration of 0.06 g/dL.
The study day timeline is outlined in Figure 1A. On the morning of the study day 1, subjects received breakfast, were re-screened for illicit drugs with a urine test, and were tested for ethanol intoxication with a breath alcohol concentration (BrAC) meter. An IV catheter was placed in an antecubital fossa vein for RAC injection and IV infusions. Approximately 30 min prior to imaging, subjects tasted 15 ml of each of the three tastants to ensure flavor recognition. Immediately preceding PET, subjects were instructed to, “Imagine you are drinking in the normal way, by taking sips of your favorite drink. Each mouse button press is the equivalent of taking a sip.” Subjects were instructed that both flavor sprays and infusion would be contingent on their response, and that their drinking progress would be shown by the three emptying glasses on the screen (Figure 1A). They were also told that ethanol infusions would be roughly equivalent to three standard drinks. Only the flavor sprays were truly contingent on subject responding, as the ethanol infusion was fixed as described below. The discrete stimulus of self-administered flavor delivery accompanied the diffuse stimulus of ascending BrAC to simulate real-world drinking stimuli. Importantly, subjects were led to believe that the alcohol was being delivered in a manner that reflected the emptying of the glasses.
While supine in the PET scanner, but before imaging, subjects responded to a “press to sip” prompt on the screen for two separate water sprays. This habituated subjects to the procedure and stimulus delivery. Subjects then rated the water to assure that they understood the subjective rating scales, and to provide a behavioral baseline. Self-administration of beer or Gatorade (delivered through a water-chilled jacket) and simultaneous infusion of ethanol or saline began precisely two minutes after RAC administration. Cartoon images of drink glasses preceded the “press to sip” and the button-press prompt. The button-press resulted in flavor delivery and a concomitant drop in the image of the fluid level of the cartoon drinking glass (which remained on-screen for 10s after response). Immediately before tracer injection, subjects were told which flavor/infusion combination they would be getting, both to minimize time spent in expectation and to avoid prediction error (Yoder et al, 2011). “Press to sip” prompts were presented in three 5-minute blocks of five prompts each; each prompt resulted in subjects responding for flavor sprays that were 750ms long, for a total of 15 sprays. Subjective ratings were acquired between blocks (i.e. after the 5th, 10th, and 15th flavor sprays; upward arrows in Figure 1A).
Gustatory stimulus delivery
Subjects self-administered flavor sprays using a mouse button, which signaled the computer-controlled “gustometer” (modeled after Marciani et al, 2006) to deliver a 0.75 ml spray. Beer, Gatorade, and water were delivered through PTFE (Teflon®) tubing via a spray nozzle, which was positioned between subject’s incisors and aimed onto the tongue. Gustometer pump motors were controlled by a PC running DASYLab software (ver. 11.0; IOtech, Inc., Cleveland, OH) with a Personal DAQ/3000 module (IOtech). On a separate laptop, E-Prime (ver. 2.0; Psychology Software Tools, Inc., Sharpsburg, PA) was configured to interface with the DASYLab software that controlled the gustometer and visual stimuli seen by subjects on a computer monitor.
Ethanol Infusion
To achieve a uniform course of BrAC in all subjects, IV ethanol (and saline) infusion profiles were individually customized using a physiologically-based pharmacokinetic model that incorporated height, weight, age and sex (O'Connor et al, 1998; Ramchandani et al, 1999). The IV ethanol infusion began simultaneously with flavor delivery (cartoon drink glasses and “press to sip” prompt), which started two minutes after RAC injection. Six percent alcohol in 0.45% saline was delivered IV by a computer-controlled peristaltic pump (iMed Gemini PC-2 TX). A nonlinear increasing rate during the 15 min “ramp” (the period of increasing BrAC to a target of 0.060 g/dL) was followed by a decreasing infusion rate during the subsequent 28 min “clamp” (the period where BrAC was held constant at 0.060 g/dL for the remainder of the scan). The pharmacokinetically-modeled ascent approximated the BrAC profile resulting from an average height/weight male consuming two to three standard beers in a 15 min period, but avoids the considerable variation associated with oral ingestion. BrACs were assessed once immediately following scan completion with a handheld breath alcohol meter (Dräger Alcotest® 6510).
Subjective Ratings
Subjects responded to computerized rating scales at four time points for each scan: once preceding the scan to establish a pre-scan baseline, and three times in the beginning of the scan at 5 min intervals (see Figure 1A). ‘Desire’ to drink alcohol was assessed with 4 items (#11, #18, #21, #32 from the Alcohol Craving Questionnaire: Singleton et al, 2000) on a 7-point visual analog scale (VAS; 1 = strongly disagree, 7 = strongly agree). Subjects were asked the number of beers they wanted at the moment, with responses in 0.5 beer increments (assuming a standard 12 oz. beer). Perceived intoxication was rated along a continuous VAS with five labels anchored by “NOT AT ALL” to “MOST EVER”. Flavor pleasantness was measured on a VAS (1 = “Least Pleasant Ever”, 7 = “Most Pleasant Ever”). Perceived flavor intensity utilized Green’s Labeled Magnitude scale (Green et al, 1996), which ranged from “barely detectable” to “strongest imaginable”. Rating scales were presented via a computer monitor, and subjects responded with a wireless mouse. Responses during the scan were averaged across time points and compared to baseline.
Image Acquisition and Processing
A 3D magnetization prepared rapid acquisition gradient echo magnetic resonance imaging (MRI) volume was acquired for all subjects using a Siemens 3T Trio-Tim (160 sagittal slices, 1.0 × 1.0 × 1.2 mm3 voxels, FOV = 256 × 256 mm, TR=2300 ms, TE = 2.91 ms, FA = 9°, duration 9:14). RAC synthesis was as described previously (Fei et al, 2004), and RAC PET scans were acquired on a Siemens EXACT HR+ (3-D mode; septa retracted). PET scans were initiated with the IV infusion of 530 ± 48 MBq RAC (mass dose 0.105 ± 0.035 nmol/kg, mean ± SD) over 1.5 min; dynamic data were acquired for 45 min (per Yoder et al, 2009).
Dynamic PET images were generated using Siemens Fourier rebinning (FORE) and filtered backprojection algorithms including corrections for attenuation, random coincidences, scattering, and dead time. Image processing utilized SPM8 (http://www.fil.ion.ucl.ac.uk/spm/) and was procedurally similar to that described previously (Oberlin et al, 2013; Yoder et al, 2012). Each subject’s anatomic MRI was used as a reference to which an early mean PET image (containing a mixture of blood flow and specific binding) was co-registered. To facilitate motion-correction, all PET frames were then co-registered to the early mean PET image (in native MRI space). After co-registration, rigid body realignment was applied to minimize spatial variance across frames and to evaluate residual motion. Each subject’s MRI was spatially transformed into Montreal Neurological Institute (MNI) space; the transformation parameters were applied to all motion-corrected dynamic PET data. A reference region was created from cerebellar gray matter, excluding the vermis (from AAL atlas; Tzourio-Mazoyer et al, 2002). Time-activity curves for the cerebellum were generated from dynamic RAC data using scripted commands (AFNI; http://afni.nimh.nih.gov/afni/). Spatially normalized D2/D3 receptor availability was indexed by binding potential (BPND), operationally defined as the bound RAC concentration relative to non-displaceable RAC concentration (Innis et al, 2007). BPND was estimated using the multilinear reference tissue model (MRTM2: Ichise et al, 2003) for all voxels within striatum, using the cerebellar time-activity curve as the input function. Only voxels with BPND values greater than 0.75 in all three scans were considered (e.g. Joutsa et al, 2012). BPND images were smoothed with a 4 mm full-width at half-maximum (FWHM) isotropic Gaussian kernel. Group averages and correlations were calculated using BPND data from each scan extracted from functional clusters defined at a height threshold of puncorr < 0.01. Mean values from these functional clusters allowed calculation of ΔBPND for each subject. Changes in BPND were expressed as a percentage of control condition, defined as:
ΔBPND = (BPND[Control] − BPND[Experimental])/BPND[Control]
with ‘Control’ and ‘Experimental’ defined as referenced in Figure 1C.
Statistics
Stimuli and Ratings
One-sample t-tests tested if BrAC values differed from the target of 0.06%; paired t-tests assessed for differences in BrAC between Gat&Eth and Beer&Eth. Delivered volume of tastants and subjective ratings were evaluated using within-subjects ANOVA across four condition levels (Pre-scan Baseline, Gat&Sal, Gat&Eth, Beer&Eth). Significant effects of Condition were followed by paired t-tests to identify differences.
Imaging
BPND images were analyzed voxelwise using a factorial model in SPM8 with three levels (Gat&Sal, Gat&Eth, Beer&Eth); peak significance was set to pFWE < 0.05, corrected for family wise error (FWE) within a search volume approximating the left or right NAcc (as defined by Neto et al, 2008). Post hoc analyses tested other factors of interest that may have influenced DA release. Mean extracted ΔBPND in responding regions (puncorr< 0.01) was calculated to assess the effects of family history (FH) and scan order among significant contrasts that emerged from the voxelwise analyses. Spearman’s rank-order correlation assessed putative relationships between ΔBPND and self-reported drinking, subjective effects, and BrAC (α = 0.05, uncorrected). To further assess the potential role of recent drinking, subjects were classified on drinks/week to Low or High groups (less or greater than the interquartile range, respectively; ns=6), and t-tests assessed differences in ΔBPND in responding regions. Laterality effects were tested with paired t-tests of mean extracted values from both the responding region and its contralateral homologous area (e.g. Wong et al, 2006). Data values in-text are presented as mean +/− standard error of the mean (SEM), where reported mean ΔBPND values illustrate effect size. Exploratory analyses were performed in caudate and putamen (defined by the AAL atlas) using the anterior commissure to delineate anterior and posterior subregions.
Results
Stimuli
Measured BrAC differed neither from target (0.059% ± 0.001 SEM and 0.060% ± 0.002 in the Gat&Eth and Beer&Eth alcohol infusion conditions, respectively; ps > 0.7) nor between conditions (p > 0.6). Tastant volume (12.2 ml ± 0.2; 12.4 ml ± 0.2; 12.1 ml ± 0.2 for Gat&Sal, Gat&Eth, and Beer&Eth scans, respectively) did not differ between conditions (p > 0.6).
Subjective Ratings
Stimulus qualities
Flavors (beer and Gatorade) were rated as more pleasant and intense than water ts(25) > 5.2, ps < 0.001, but the flavors did not differ from each other (ps > 0.2).
Desire to drink
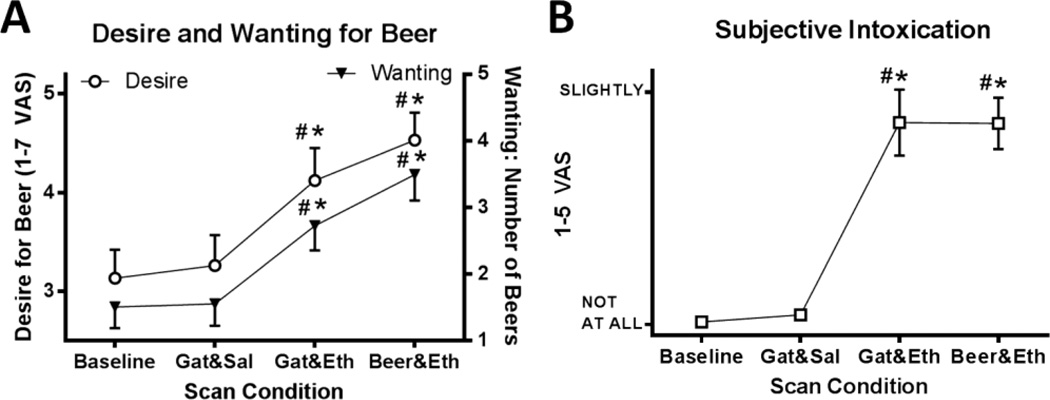
The ethanol conditions increased both Desire and Wanting relative to baseline and the Gat&Sal condition, [ANOVA; F(3,75) = 18.8, p < 0.001, paired-t; ts(25) > 3.4, ps ≤ 0.002]. The ethanol conditions did not differ in Desire (p = 0.09), but Beer&Eth showed a trend to increase number of beers wanted when compared to Gat&Eth (p = 0.052; Figure 2A).
Fig. 2.
Subjective States. (A) All 26 subjects rated the desire for beer (open circles, left y-axis), and the number of beers wanted (filled triangles, right y-axis) with saline or alcohol infusion. (B) Subjects rated their subjective state of intoxication (open squares). Mean ± SEM are presented; # = differed from water Baseline, * = differed from Gatorade-Saline; (p < 0.05). The Baseline condition was evaluated after two water sprays and prior to each flavor-infusion scan; these are averaged across flavor conditions. VAS = Visual Analog Scale, Gat = Gatorade flavor, Sal = saline infusion; Alc = alcohol infusion.
Subjective intoxication
Ethanol infusions increased perceived intoxication relative to baseline and Gat&Sal, [ANOVA; F(3,75) = 34.7, p < 0.001, paired-t; ts(25) > 5.6, ps < 0.001], but ethanol infusions did not differ from each other (p > 0.9; Figure 2B).
Imaging: stimulus effects
Effects of ethanol
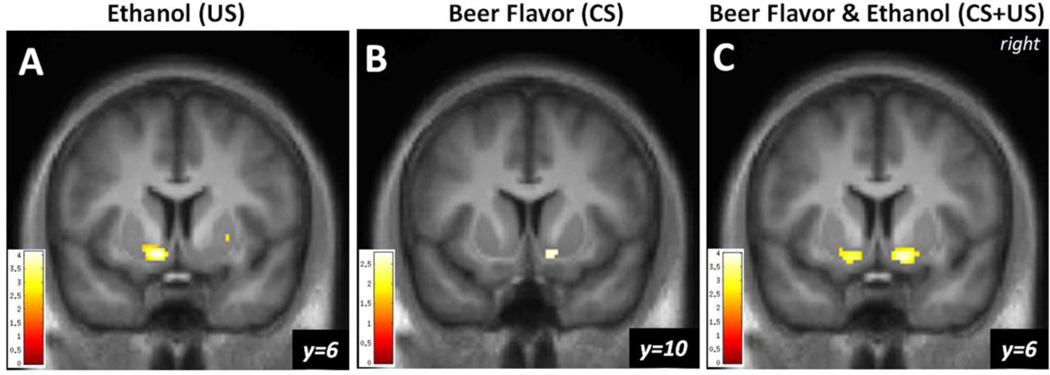
Compared to Gat&Sal, Gat&Eth significantly reduced left NAcc RAC BPND, indicating higher DA in the alcohol condition (n = 26, peak voxel [−10, 6, −10], Z = 3.91, pFWE = 0.001; Figure 3A). ΔBPND (percent change) within the responding cluster was 5.9% ± 0.8%. There was no effect in R NAcc.
Fig. 3.
(A) DA response to ethanol, with Gatorade flavor. Statistical map illustrating the nucleus accumbens (NAcc) DA response to ethanol infusion with Gatorade flavor [Gat&Sal BPND > Gat&Eth BPND] in male drinkers (n = 26) as indexed by [11C]raclopride displacement. (B) DA response to beer flavor, with ethanol. The NAcc response to beer flavor relative to Gatorade, both under ethanol, [Gat&Eth BPND > Beer&Eth BPND]. (C) DA response to beer flavor plus ethanol. The NAcc response to beer flavor and ethanol relative to Gatorade and saline, [Gat&Sal BPND > Beer&Eth BPND]. The color bar shows the voxel-wise t statistic. Display threshold at p < 0.01, uncorrected, and k = 20. All results displayed, pFWE < 0.05, after correction for NAcc search volumes.
Effects of beer flavor
Compared to Gat&Eth, Beer&Eth significantly reduced RAC BPND (increased DA in response to beer flavor) in the right NAcc (peak voxel [12, 10, −10], Z = 2.71, pFWE = 0.043, Figure 3B). ΔBPND within the responding cluster was 6.8% ± 2.6%. There was no effect in L NAcc.
Effects of beer flavor plus alcohol
Compared to the baseline of Gat&Sal, the combined exposure to both beer flavor and ethanol intoxication (i.e., Beer&Eth) significantly reduced RAC BPND (increased DA) in both left ([−14, 6, −12], Z = 3.19, pFWE = 0.013) and right [12, 8, −10], Z = 3.75, pFWE = 0.002; Figure 3C) NAcc. Average ΔBPND was 5.8% ± 1.5% and 6.8% ± 2.2% for the responding L and R clusters, respectively.
Imaging: factors modulating DA response
Perceived intoxication
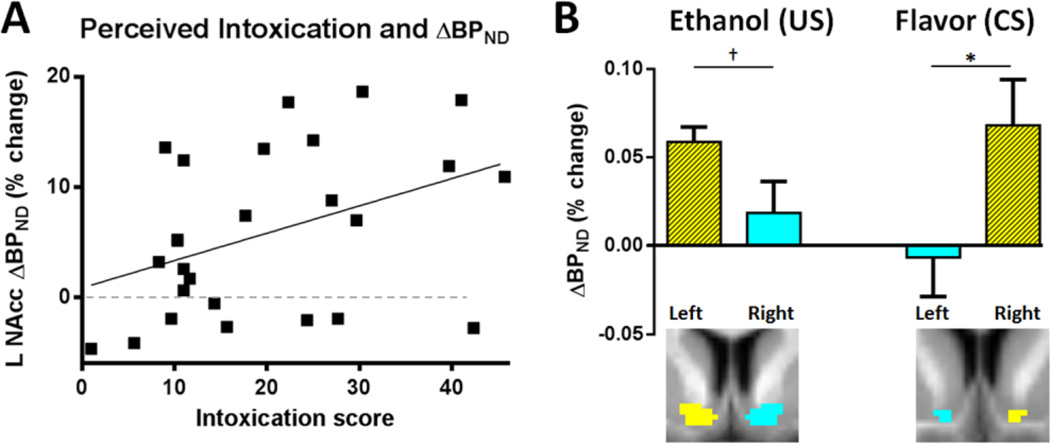
Ethanol plus flavor-induced displacement of RAC in L NAcc (Beer&Eth vs. Gat&Sal) correlated positively with perceived intoxication (rho = 0.399, p = 0.044; Figure 4A), with higher perceived intoxication corresponding to greater DA response to alcohol plus beer flavor. No correlation was observed on the right. Self-reported intoxication did not correlate with L NAcc DA response in the Gat&Eth vs. Gat&Sal comparison.
Fig. 4.
(A) DA Effects of ethanol and subjective response. In all subjects (n = 26), self-reported intoxication correlated with increased displacement of [11C]raclopride (ΔBPND) to ethanol with beer flavor in L NAcc, as illustrated in Fig 3A; rho = 0.399, p = 0.044. The dashed line indicates unchanged ΔBPND. (B) Laterality. Mean reduction in BPND in response to ethanol and beer flavor (shown in Figs 3A,B) showed a lateralized response, as compared to the homologous region. Columns and spatial extent of responding regions shown in (hatched) yellow, with homologous regions (coronal view, below) indicated in cyan. *p < 0.05, †p = 0.057
BrAC
Measured BrAC in the Gat&Eth scan showed a trend-level correlation with ΔBPND in L NAcc in the Gat&Eth vs. Gat&Sal comparison (rho = 0.361, p = 0.070). No other correlations with BrAC were detected.
Family history
As the FH groups differed unexpectedly in BrAC, ts(24) > 3.4, ps < 0.003, FH tests of ΔBPND in each responding region included a BrAC covariate. There were no differences in ΔBPND by FH group in any outcome (ps > 0.19).
Order effects
ΔBPND did not differ based on which condition was presented first, i.e. Beer&Eth or Gat&Eth, in any of the responding regions (ps > 0.10).
Other factors
Subjective ratings of flavor pleasantness, intensity, desire, or wanting for alcohol did not correlate with ΔBPND in any of the responding regions in their respective contrasts, i.e. L NAcc response to the Gat&Eth vs. Gat&Sal comparison, the R NAcc response to the Beer&Eth vs. Gat&Eth comparison, or in either the L or R NAcc response to the Beer&Eth vs. Gat&Sal comparison. No correlations of ΔBPND with age, age of first or regular drinking, or recent drinking frequency were observed. Low and High drinker groups did not differ in responding regions (ps > 0.27).
Imaging: other analyses
Laterality
The R NAcc region responding to flavor, i.e. [Gat&Eth > Beer&Eth], showed a larger DA response than the corresponding region on the left, t(25) = 2.2, p = 0.037. The L NAcc region responding to intoxication, i.e. [Gat&Sal > Gat&Eth], showed a strong trend of difference (p = 0.057) compared to the homologous area on the right; Figure 4B.
Gatorade flavor
The inverse contrasts to those reported above (i.e. Gat&Sal BPND vs. Gat&Eth BPND, DA release to Gatorade), showed no significant effects in either L or R NAcc.
Extra-NAcc effects
Exploratory analyses of dorsal anterior and posterior caudate and putamen did not show significant effects in any contrast at the threshold pFWE < 0.05, corrected by region.
Discussion
Utilizing a novel analog of self-administration paradigm in heavy drinkers that separates ethanol’s conditioned sensory properties from intoxication, we found that relative increases in NAcc DA transmission were localized to the right side for an alcohol-related CS (flavor) and to the left for the US (intoxication). This accords with our previous finding that beer flavor alone increased right NAcc DA in a similar population of heavy drinkers (Oberlin et al, 2013), and that (unexpected) intravenous ethanol intoxication increased left VST DA (Yoder et al, 2009). Previous reports showed bilateral VST DA increases resulting from ethanol CS and US presented together (Boileau et al, 2003; Setiawan et al, 2014; Urban et al, 2010), although in these studies, fruit juice was the tastant (not necessarily the preferred mixer), meaning that ethanol’s orosensory properties could have acted as a CS. If we regard these previous studies as concomitant alcohol CS and US presentation, these data comport with our current findings of bilateral NAcc DA release to combined alcohol CS and US presentation. We can thus infer that the DA response to naturalistic alcohol drinking, which includes CS and US presentation, is evident in bilateral NAcc responses, but with lateralized processing of stimulus types.
Although interpreting lateralized DA responses to alcohol CS and US as separate information processing streams has an attractive simplicity, an alternate conceptualization is that both cue-reactivity and DA responses to intoxication are conditioned responses. For example, there is considerable evidence in animals showing that the US of a drug can reinstate (conditioned) drug-seeking behaviors (for review, see Epstein et al, 2006), even when the US is an entirely different drug; that is, multiple drugs are capable of reinstating cocaine or heroin responding (de Wit and Stewart, 1981, 1983). This implies that internal state changes induced by a drug of abuse acquire properties that act as discriminative stimuli which motivate behavior (Gerber and Stretch, 1975). Studies reveal that reinstatement depends, at least in part, on intact DA functioning (Alleweireldt et al, 2002; Xi et al, 2006). Similarly, conditioned (discriminative) stimuli that are paired with drug availability and seeking also motivate drug-taking behavior, and appear to depend on DA function (Bossert et al, 2007). As both internal and external stimuli can become conditioned to particular effects of a drug, a wide array of conditioning possibilities exist such that the line between a conditioned response and the effects from a US becomes blurred, that is, both can act as discriminative stimuli. Given that drug-paired CS and drug US both activate appetitive motivational mechanisms, which increase motivated behaviors, they also both fit the classical definition of a reinforcer. In this light, lateralized conditioned responses may simply reflect specialized sensitivity to the nature of the drug stimuli, such that the R NAcc is more sensitive to conditioned cues (flavor) signaling impending intoxication, while L NAcc may be more differentially sensitive to the perceived salient changes in internal states brought on by intoxication, and learned through experience (e.g., reward signaling, dizziness).
This interpretation is consistent with a previous study (Besson and Louilot, 1995) that utilized in vivo voltammetry and a conditioned taste aversion paradigm in which a flavor was conditioned to a nausea-inducing lithium salt. The CS that predicted nausea reduced L NAcc DA transmission (suggesting that L NAcc DA responds to internal state changes, and perhaps valence), while the R NAcc showed a stronger response to an appetitive (external) stimulus. Importantly, both responses had been learned (conditioned) (Besson et al, 1995). The synthesis of these prior data and the current findings suggests that NAcc DA may instantiate learned responses in a lateralized fashion, such that L NAcc is more linked to signaling interoceptive changes, while R NAcc signals salient exteroceptive stimuli.
The R NAcc response, which was relatively weak when both beer and control flavors were compared under ethanol (Beer&Eth vs. Gat&Eth), responded robustly in conjunction with the left NAcc when beer flavor under ethanol intoxication was compared to Gatorade with saline (i.e., the combination of stimuli closest to naturalistic alcohol ‘drinking’). These DA responses support the notion that intoxication is additive to DA effects induced by the CS of an alcoholic drink’s characteristic flavor, perhaps due to the presence of the additional CS of internal state changes. Further evidence for this type of stimulus synergy is observed in the correlation of subjective intoxication with ΔBPND in L NAcc, which was only detectable when beer flavor was present. This finding was not replicated on the right, implicating a greater relative contribution of sensitivity to interoceptive effects on the left.
Viewing the effect of intoxication as a learned response comports with previous data suggesting that a history of heavy drinking may be needed for ethanol’s ability to induce VST DA release, as DA response was not observed in social drinkers (Yoder et al, 2007; Yoder et al, 2005), or in social drinkers with the major (common) allele of the functional μ-opioid receptor gene, OPRM1 (Ramchandani et al, 2011). Notably, the subjects of Urban et al. (2010) study drank more heavily than typical social drinkers, and showed DA release to orally consumed ethanol. Given the lack of correlation in drinking history with DA response, or even a difference between extremes of drinking in this sample (which presumably differ in conditioning history), the effect of conditioning may not be strictly linear, but instead reflects a threshold of conditioning. If true, this conditioning might be formed after a very few intoxication sessions; alternatively, a heightened capacity for a DA conditioned response to intoxication might be mediated by other factors—additional studies will be needed to inform these hypotheses.
Other studies have shown lateralized striatal effects that suggest specialization by hemisphere. Congruent with the idea that R striatal DA signaling may be more sensitive to CS, Wong et al. (2006) reported that cocaine addicts (‘cravers’) showed greater R dorsal putamen DA release to audiovisual cues than non-cravers. A study in healthy subjects revealed that R VST DA preferentially responded to unexpected monetary rewards relative to L VST (Martin-Soelch et al, 2011). Another study in healthy controls showed that incentive motivation correlated with resting D2 receptor availability in the left putamen; an interpretation of these data is that greater endogenous right putamen DA (relative to left putamen) predicts higher drive (Tomer et al, 2008). In Parkinson’s patients, right hemisphere DA deficits corresponded with reduced novelty-seeking, relative to controls (Tomer and Aharon-Peretz, 2004), also suggesting lateralized striatal DA function.
While interpretations differ as to the meaning of mesolimbic DA responses, it is clear that alcohol and other drugs of abuse potentiate DA release in the midbrain (e.g., Brodie et al, 1990) and NAcc (Di Chiara et al, 1988; Imperato and Di Chiara, 1986; Sullivan et al, 2011). NAcc DA may also be necessary for CS-induced drug-seeking behavior, as local infusion of a DA receptor antagonist in NAcc reduces both alcohol seeking and consumption (Czachowski et al, 2001); similarly, the ability of alcohol’s contextual cues to induce alcohol-seeking is blocked by DA receptor antagonists (Hamlin et al, 2007). Renewal-induced transcription factor induction (c-Fos) in NAcc requires normal DA D1 receptor function, suggesting that NAcc DA modulates long-term changes in NAcc gene expression specific to alcoholseeking behavior (Hamlin et al, 2007), and providing a possible mechanism for learned responses in NAcc.
Some limitations to this work should be considered. While the paradigm was truly CS self-administration, it was not drug self-administration in the strictest sense. From the standpoint of expectations, the paradigm was self-administration in the fullest sense as subjects were instructed that infusion depended on their responses. Importantly, this method captured the essence of any traditional self-administration paradigm: the presence of an instrumental response for more drug during a period of increasing drug plasma concentration. The most conservative statistical correction for the imaging data would employ bilateral NAcc search regions. Analyzed in this way, all but one of our results remained significant (psFWE < 0.05). Only the effect of beer flavor in the R NAcc (which replicates our earlier finding in a much larger sample; Oberlin et al, 2013) became a trend-level result (pFWE = 0.086). Family history of alcoholism may also influence how subjects response to alcohol CS and US. However, we did not find family history differences in DA responses in the current study. Although this is discrepant with our prior data (Oberlin et al, 2013), this sample is somewhat smaller, and the family history effects of our earlier report occurred to a flavor CS without concomitant ethanol intoxication or operant requirement (see Kareken et al, 2010 for attenuation of conditioned responses by alcohol in FHP). We also note that at least 8 subjects were non-responders in the L NAcc to the Beer&Eth condition. In combination with the lack of relationship between recent drinking history and DA release, this heterogeneity of DA response suggests that other important factors might be mediating DA processing of alcohol stimuli. Larger samples will likely be required to characterize these factors.
In summary, heavy beer drinkers showed increased VST DA release on the right to beer flavor, on the left to intoxication, and bilaterally in a combination that mimicked drinking as it occurs naturalistically (CS plus US). These data show that, within the same individuals, the independent components of an alcoholic drink have additive and lateralized effects on VST DA release, and that these effects may reflect interoceptive and exteroceptive stimulus processing.
Acknowledgements
We gratefully acknowledge Kevin Perry, Wendy Territo, Michele Beal, Courtney Robbins (Dept. of Radiology and Imaging Sciences), Dr. William Eiler, Traci Mitchell (Dept. of Neurology) for technical assistance, and Dwight Hector for design and construction of the gustometer.
Supported by R01 AA017661 (DAK), the Indiana Alcohol Research Center P60 AA007611 for additional support to DAK; TR000006 (Indiana Clinical and Translational Sciences Institute, Clinical Research Center at Indiana University School of Medicine), T32 AA007462 (BGO), and R01 AA018354 (KKY).
Footnotes
The authors declare no conflict of interest.
Authors maintain full control of all primary data and agree to review by journal upon request.
References
- Alleweireldt AT, Weber SM, Kirschner KF, Bullock BL, Neisewander JL. Blockade or stimulation of D1 dopamine receptors attenuates cue reinstatement of extinguished cocaine-seeking behavior in rats. Psychopharmacology (Berl) 2002;159(3):284–293. doi: 10.1007/s002130100904. [DOI] [PubMed] [Google Scholar]
- Besson C, Louilot A. Asymmetrical involvement of mesolimbic dopaminergic neurons in affective perception. Neuroscience. 1995;68(4):963–968. doi: 10.1016/0306-4522(95)00255-h. [DOI] [PubMed] [Google Scholar]
- Bjork JM, Hommer DW. Anticipating instrumentally obtained and passively-received rewards: a factorial fMRI investigation. Behav Brain Res. 2007;177(1):165–170. doi: 10.1016/j.bbr.2006.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boileau I, Assaad JM, Pihl RO, Benkelfat C, Leyton M, Diksic M, et al. Alcohol promotes dopamine release in the human nucleus accumbens. Synapse. 2003;49(4):226–231. doi: 10.1002/syn.10226. [DOI] [PubMed] [Google Scholar]
- Bossert JM, Poles GC, Wihbey KA, Koya E, Shaham Y. Differential effects of blockade of dopamine D1-family receptors in nucleus accumbens core or shell on reinstatement of heroin seeking induced by contextual and discrete cues. J Neurosci. 2007;27(46):12655–12663. doi: 10.1523/JNEUROSCI.3926-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodie MS, Shefner SA, Dunwiddie TV. Ethanol increases the firing rate of dopamine neurons of the rat ventral tegmental area in vitro. Brain Res. 1990;508(1):65–69. doi: 10.1016/0006-8993(90)91118-z. [DOI] [PubMed] [Google Scholar]
- Bucholz KK, Cadoret R, Cloninger CR, Dinwiddie SH, Hesselbrock VM, Nurnberger JI, Jr, et al. A new, semi-structured psychiatric interview for use in genetic linkage studies: a report on the reliability of the SSAGA. J Stud Alcohol. 1994;55(2):149–158. doi: 10.15288/jsa.1994.55.149. [DOI] [PubMed] [Google Scholar]
- Crombag HS, Bossert JM, Koya E, Shaham Y. Review. Context-induced relapse to drug seeking: a review. Philos Trans R Soc Lond B Biol Sci. 2008;363(1507):3233–3243. doi: 10.1098/rstb.2008.0090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czachowski CL, Chappell AM, Samson HH. Effects of raclopride in the nucleus accumbens on ethanol seeking and consumption. Alcohol Clin Exp Res. 2001;25(10):1431–1440. doi: 10.1097/00000374-200110000-00005. [DOI] [PubMed] [Google Scholar]
- de Wit H, Stewart J. Reinstatement of cocaine-reinforced responding in the rat. Psychopharmacology (Berl) 1981;75(2):134–143. doi: 10.1007/BF00432175. [DOI] [PubMed] [Google Scholar]
- de Wit H, Stewart J. Drug reinstatement of heroin-reinforced responding in the rat. Psychopharmacology (Berl) 1983;79(1):29–31. doi: 10.1007/BF00433012. [DOI] [PubMed] [Google Scholar]
- Di Chiara G, Imperato A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc Natl Acad Sci U S A. 1988;85(14):5274–5278. doi: 10.1073/pnas.85.14.5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyon WM, Anders SK, Ramachandra VS, Czachowski CL, Gonzales RA. Effect of operant selfadministration of 10% ethanol plus 10% sucrose on dopamine and ethanol concentrations in the nucleus accumbens. J Neurochem. 2005;93(6):1469–1481. doi: 10.1111/j.1471-4159.2005.03137.x. [DOI] [PubMed] [Google Scholar]
- Epstein DH, Preston KL, Stewart J, Shaham Y. Toward a model of drug relapse: an assessment of the validity of the reinstatement procedure. Psychopharmacology (Berl) 2006;189(1):1–16. doi: 10.1007/s00213-006-0529-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fei X, Mock BH, DeGrado TR, Wang JQ, Glick-Wilson BE, Sullivan ML, et al. An improved synthesis of PET dopamine D2 receptors radioligand [11C]raclopride. Synthetic Communications. 2004;34(10) [Google Scholar]
- Gerber GJ, Stretch R. Drug-induced reinstatement of extinguished self-administration behavior in monkeys. Pharmacol Biochem Behav. 1975;3(6):1055–1061. doi: 10.1016/0091-3057(75)90016-7. [DOI] [PubMed] [Google Scholar]
- Grant S, London ED, Newlin DB, Villemagne VL, Liu X, Contoreggi C, et al. Activation of memory circuits during cue-elicited cocaine craving. Proc Natl Acad Sci U S A. 1996;93(21):12040–12045. doi: 10.1073/pnas.93.21.12040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green BG, Dalton P, Cowart B, Shaffer G, Rankin K, Higgins J. Evaluating the 'Labeled Magnitude Scale' for measuring sensations of taste and smell. Chem Senses. 1996;21(3):323–334. doi: 10.1093/chemse/21.3.323. [DOI] [PubMed] [Google Scholar]
- Hamlin AS, Newby J, McNally GP. The neural correlates and role of D1 dopamine receptors in renewal of extinguished alcohol-seeking. Neuroscience. 2007;146(2):525–536. doi: 10.1016/j.neuroscience.2007.01.063. [DOI] [PubMed] [Google Scholar]
- Ichise M, Liow JS, Lu JQ, Takano A, Model K, Toyama H, et al. Linearized reference tissue parametric imaging methods: application to [11C]DASB positron emission tomography studies of the serotonin transporter in human brain. J Cereb Blood Flow Metab. 2003;23(9):1096–1112. doi: 10.1097/01.WCB.0000085441.37552.CA. [DOI] [PubMed] [Google Scholar]
- Imperato A, Di Chiara G. Preferential stimulation of dopamine release in the nucleus accumbens of freely moving rats by ethanol. J Pharmacol Exp Ther. 1986;239(1):219–228. [PubMed] [Google Scholar]
- Innis RB, Cunningham VJ, Delforge J, Fujita M, Gjedde A, Gunn RN, et al. Consensus nomenclature for in vivo imaging of reversibly binding radioligands. J Cereb Blood Flow Metab. 2007;27(9):1533–1539. doi: 10.1038/sj.jcbfm.9600493. [DOI] [PubMed] [Google Scholar]
- Joutsa J, Johansson J, Niemela S, Ollikainen A, Hirvonen MM, Piepponen P, et al. Mesolimbic dopamine release is linked to symptom severity in pathological gambling. Neuroimage. 2012;60(4):1992–1999. doi: 10.1016/j.neuroimage.2012.02.006. [DOI] [PubMed] [Google Scholar]
- Kareken DA, Bragulat V, Dzemidzic M, Cox C, Talavage T, Davidson D, et al. Family history of alcoholism mediates the frontal response to alcoholic drink odors and alcohol in at-risk drinkers. Neuroimage. 2010;50(1):267–276. doi: 10.1016/j.neuroimage.2009.11.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marciani L, Pfeiffer JC, Hort J, Head K, Bush D, Taylor AJ, et al. Improved methods for fMRI studies of combined taste and aroma stimuli. J Neurosci Methods. 2006;158(2):186–194. doi: 10.1016/j.jneumeth.2006.05.035. [DOI] [PubMed] [Google Scholar]
- Martin-Soelch C, Szczepanik J, Nugent A, Barhaghi K, Rallis D, Herscovitch P, et al. Lateralization and gender differences in the dopaminergic response to unpredictable reward in the human ventral striatum. Eur J Neurosci. 2011;33(9):1706–1715. doi: 10.1111/j.1460-9568.2011.07642.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neto LL, Oliveira E, Correia F, Ferreira AG. The human nucleus accumbens: where is it? A stereotactic, anatomical and magnetic resonance imaging study. Neuromodulation : journal of the International Neuromodulation Society. 2008;11(1):13–22. doi: 10.1111/j.1525-1403.2007.00138.x. [DOI] [PubMed] [Google Scholar]
- O'Connor S, Morzorati S, Christian J, Li TK. Clamping breath alcohol concentration reduces experimental variance: application to the study of acute tolerance to alcohol and alcohol elimination rate. Alcohol Clin Exp Res. 1998;22(1):202–210. [PubMed] [Google Scholar]
- Oberlin BG, Dzemidzic M, Tran SM, Soeurt CM, Albrecht DS, Yoder KK, et al. Beer flavor provokes striatal dopamine release in male drinkers: mediation by family history of alcoholism. Neuropsychopharmacology. 2013;38(9):1617–1624. doi: 10.1038/npp.2013.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips PE, Stuber GD, Heien ML, Wightman RM, Carelli RM. Subsecond dopamine release promotes cocaine seeking. Nature. 2003;422(6932):614–618. doi: 10.1038/nature01476. [DOI] [PubMed] [Google Scholar]
- Ramchandani VA, Bolane J, Li TK, O'Connor S. A physiologically-based pharmacokinetic (PBPK) model for alcohol facilitates rapid BrAC clamping. Alcohol Clin Exp Res. 1999;23(4):617–623. [PubMed] [Google Scholar]
- Ramchandani VA, Umhau J, Pavon FJ, Ruiz-Velasco V, Margas W, Sun H, et al. A genetic determinant of the striatal dopamine response to alcohol in men. Mol Psychiatry. 2011;16(8):809–817. doi: 10.1038/mp.2010.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Brain Res Rev. 1993;18(3):247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. Review. The incentive sensitization theory of addiction: some current issues. Philos Trans R Soc Lond B Biol Sci. 2008;363(1507):3137–3146. doi: 10.1098/rstb.2008.0093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchis-Segura C, Spanagel R. Behavioural assessment of drug reinforcement and addictive features in rodents: an overview. Addict Biol. 2006;11(1):2–38. doi: 10.1111/j.1369-1600.2006.00012.x. [DOI] [PubMed] [Google Scholar]
- Saunders JB, Aasland OG, Babor TF, de la Fuente JR, Grant M. Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO Collaborative Project on Early Detection of Persons with Harmful Alcohol Consumption--II. Addiction. 1993;88(6):791–804. doi: 10.1111/j.1360-0443.1993.tb02093.x. [DOI] [PubMed] [Google Scholar]
- Schultz W, Dayan P, Montague PR. A neural substrate of prediction and reward. Science. 1997;275(5306):1593–1599. doi: 10.1126/science.275.5306.1593. [DOI] [PubMed] [Google Scholar]
- Setiawan E, Pihl RO, Dagher A, Schlagintweit H, Casey KF, Benkelfat C, et al. Differential striatal dopamine responses following oral alcohol in individuals at varying risk for dependence. Alcohol Clin Exp Res. 2014;38(1):126–134. doi: 10.1111/acer.12218. [DOI] [PubMed] [Google Scholar]
- Singleton EG, Tiffany ST, Henningfield JE. Alcohol Craving Questionnaire (ACQ-NOW): Background, Scoring, and Administration. Baltimore, MD: Intramural Research Program, National Institute on Drug Abuse; 2000. [Google Scholar]
- Sobell MB, Sobell LC, Klajner F, Pavan D, Basian E. The reliability of a timeline method for assessing normal drinker college students' recent drinking history: utility for alcohol research. Addict Behav. 1986;11(2):149–161. doi: 10.1016/0306-4603(86)90040-7. [DOI] [PubMed] [Google Scholar]
- Sullivan JM, Risacher SL, Normandin MD, Yoder KK, Froehlich JC, Morris ED. Imaging of alcohol-induced dopamine release in rats:preliminary findings with [(11) C]raclopride PET. Synapse. 2011;65(9):929–937. doi: 10.1002/syn.20921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiffany ST. Cognitive concepts of craving. Alcohol Res Health. 1999;23(3):215–224. [PMC free article] [PubMed] [Google Scholar]
- Tomer R, Aharon-Peretz J. Novelty seeking and harm avoidance in Parkinson's disease: effects of asymmetric dopamine deficiency. J Neurol Neurosurg Psychiatry. 2004;75(7):972–975. doi: 10.1136/jnnp.2003.024885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomer R, Goldstein RZ, Wang GJ, Wong C, Volkow ND. Incentive motivation is associated with striatal dopamine asymmetry. Biol Psychol. 2008;77(1):98–101. doi: 10.1016/j.biopsycho.2007.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15(1):273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- Urban NB, Kegeles LS, Slifstein M, Xu X, Martinez D, Sakr E, et al. Sex differences in striatal dopamine release in young adults after oral alcohol challenge: a positron emission tomography imaging study with [(1)(1)C]raclopride. Biol Psychiatry. 2010;68(8):689–696. doi: 10.1016/j.biopsych.2010.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss F, Lorang MT, Bloom FE, Koob GF. Oral alcohol self-administration stimulates dopamine release in the rat nucleus accumbens: genetic and motivational determinants. J Pharmacol Exp Ther. 1993;267(1):250–258. [PubMed] [Google Scholar]
- Wong DF, Kuwabara H, Schretlen DJ, Bonson KR, Zhou Y, Nandi A, et al. Increased occupancy of dopamine receptors in human striatum during cue-elicited cocaine craving. Neuropsychopharmacology. 2006;31(12):2716–2727. doi: 10.1038/sj.npp.1301194. [DOI] [PubMed] [Google Scholar]
- Xi ZX, Newman AH, Gilbert JG, Pak AC, Peng XQ, Ashby CR, Jr, et al. The novel dopamine D3 receptor antagonist NGB 2904 inhibits cocaine's rewarding effects and cocaine-induced reinstatement of drug-seeking behavior in rats. Neuropsychopharmacology. 2006;31(7):1393–1405. doi: 10.1038/sj.npp.1300912. [DOI] [PubMed] [Google Scholar]
- Yoder KK, Albrecht DS, Kareken DA, Federici LM, Perry KM, Patton EA, et al. Reliability of striatal [11C]raclopride binding in smokers wearing transdermal nicotine patches. Eur J Nucl Med Mol Imaging. 2012;39(2):220–225. doi: 10.1007/s00259-011-1965-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoder KK, Constantinescu CC, Kareken DA, Normandin MD, Cheng TE, O'Connor SJ, et al. Heterogeneous effects of alcohol on dopamine release in the striatum: a PET study. Alcohol Clin Exp Res. 2007;31(6):965–973. doi: 10.1111/j.1530-0277.2007.00390.x. [DOI] [PubMed] [Google Scholar]
- Yoder KK, Kareken DA, Morris ED. Assessing dopaminergic neurotransmission with PET: basic theory and applications in alcohol research. Current Medical Imaging Reviews. 2011;7:118–124. [Google Scholar]
- Yoder KK, Kareken DA, Seyoum RA, O'Connor SJ, Wang C, Zheng QH, et al. Dopamine D(2) receptor availability is associated with subjective responses to alcohol. Alcohol Clin Exp Res. 2005;29(6):965–970. doi: 10.1097/01.alc.0000171041.32716.42. [DOI] [PubMed] [Google Scholar]
- Yoder KK, Morris ED, Constantinescu CC, Cheng TE, Normandin MD, O'Connor SJ, et al. When what you see isn't what you get: alcohol cues, alcohol administration, prediction error, and human striatal dopamine. Alcohol Clin Exp Res. 2009;33(1):139–149. doi: 10.1111/j.1530-0277.2008.00821.x. [DOI] [PMC free article] [PubMed] [Google Scholar]