Abstract
We hypothesize upright cognitive impairment in Postural Tachycardia Syndrome is due to reduced cerebral blood flow. Cerebral blood flow velocity measured by transcranial Doppler ultrasound decreased excessively during 70° tilt in a minority of patients with intermittent hyperpnea/hypocapnia. Incremental tilt showed no difference in mean cerebral blood flow velocity. But, N-Back memory tasking indicated progressive compromised memory, reduced functional hyperemia and reduced neurovascular coupling. Orthostasis caused slow oscillations in cerebral blood flow velocity linked to oscillations in arterial pressure in Postural Tachycardia Syndrome. We also hypothesize that oscillatory cerebral blood flow velocity degrades neurovascular coupling. We performed 2-Back testing supine and during incremental tilts to 15°, 30°, 45° and 60° in 11 Postural Tachycardia Syndrome and 9 controls. Oscillatory arterial pressure, oscillatory cerebral blood flow velocity and neurovascular coupling were similar supine. Oscillatory arterial pressure increased 31, 45, 67, and 93% in Postural Tachycardia Syndrome during tilt, remaining unchanged in control. Oscillatory cerebral blood flow velocity increased by 61, 82, 161, and 264% in Postural Tachycardia Syndrome during tilt remaining unchanged in control. Functional hyperemia decreased from 4.1% to 3.0, 1.1, 0.2, to 0.04% in Postural Tachycardia Syndrome but was unchanged at 4% in control. Percent correct N-Back responses decreased from 78% to 33% in Postural Tachycardia Syndrome while remaining at 89% in controls. In Postural Tachycardia Syndrome, oscillatory cerebral blood flow velocity was linearly correlated with functional hyperemia (r2=0.76). Increased oscillatory cerebral blood flow is associated with reduced neurovascular coupling and diminished cognitive performance in Postural Tachycardia Syndrome.
Keywords: Orthostatic intolerance, cerebral autoregulation, cognition, neurovascular coupling, oscillations
Introduction
Orthostatic intolerance (OI) is defined by signs and symptoms of lightheadedness, tachycardia, diaphoresis, heat, hypotension, hyperpnea, headache, nausea, fatigue, cognitive deficits, and exercise intolerance while upright relieved by recumbence 1;2. Postural Tachycardia Syndrome (POTS) is chronic OI associated with excess upright tachycardia without hypotension 3-7. POTS patients often report “Brain Fog” while upright to describe impaired awareness, mental confusion, lightheadedness, mental fatigue, and cognitive deficits, especially of working memory 8.
We initially hypothesized that Brain Fog is due to orthostatic reductions of cerebral blood flow (CBF) impairing neuronal activation 9. Although CBF decreases excessively in POTS compared to controls 10, we later showed mean CBF was abnormally reduced only in some during rapid orthostasis 11. In these patients, large reductions in CBF occurred intermittently, and in response to a rapid initially decreased central blood volume 11. Excessively reduced CBF does not occur in most POTS patients, although Brain Fog is consistently present. Thus, reduced CBF is not a prerequisite for orthostasis-induced diminished central nervous system function.
Changes in CBF do not explain Brain Fog as decreased CBF did not occur with incremental upright tilts, and mean CBF, while decreasing with tilt angle, was similar for POTS and controls 12. Here we used N-Back tasking to quantitate working memory, concentration, and information processing of progressive difficulty 13. We combined N-Back with transcranial Doppler ultrasound (TCD) of the middle cerebral artery (MCA) during incremental tilt. During N-Back, velocity of CBF (CBFv) in the MCA increases above the mean in control subjects 14 in the absence of blood pressure or ETCO2 changes15, shown in Figure S1 in the online-only Data Supplement. This increase is called “functional” or “neural activity related hyperemia”. The relation between neural activity and functional hyperemia is called neurovascular coupling (NVC) and involves interactions among components of the neurovascular unit16.
The accuracy of N-Back responses and functional hyperemia deteriorated with tilt angle in POTS but not controls 12, signifying that progressive orthostatic stress impairs cognitive performance and neurovascular coupling. Decreased functional hyperemia and blunted NVC 17 may therefore result in cognitive dysfunction in POTS.
We recently observed greatly increased slow CBFv oscillations (<0.40 Hz) in POTS compared to controls during 70° upright tilt 18 suggesting that oscillatory CBF power interferes with cognition and neurovascular coupling. Therefore, we used incremental upright tilt and related increases in OCBF to decreases of functional hyperemia and cognitive performance in POTS, but not in healthy volunteers.
Methods
Outline
We tested neurocognition during incremental tilt by administering an N-Back memory task to measure executive working memory, concentration, and information processing 13;14. We used a 2-Back memory task which best discriminated between control and POTS in previous work 12. CBFv of the left MCA, which assessed the functional hyperemic response during 2-Back cognitive activation14;19;20, was measured at 0°, 15°, 30°, 45°, 60°; 75° was not used because it resulted in vasovagal syncope in POTS and controls.
Subjects
We enrolled 11 POTS subjects 18-26 years old (median age 22.3 yrs, 9 female, 2 males) with POTS defined by standard criteria5. All had symptoms for >6 months. POTS was identified during a separate tilt to 70° by signs and symptoms of OI and excessive increase in heart rate (HR) without hypotension within 10 minutes of head-up tilt (HUT) 6;21;22. Medical problems that could explain these signs or symptoms had been previously ruled out.
Nine healthy volunteers were enrolled as controls aged 17-27 yrs old (median age 21.4, 6 female, 3 male); non-smokers with no previously known medical conditions or illness, taking no medications, with normal physical exams and electrocardiograms. Healthy control subjects never experienced OI, including orthostatic hypotension, POTS or syncope. All refrained from medications for at least 2 weeks prior to study except for contraceptives, and stopped xanthine-, caffeine-, or alcohol-containing substances 72 hours prior to study.
The New York Medical College IRB reviewed and approved this protocol. Each subject received a detailed description of all protocols. Signed informed consent was obtained from all participants or their parents.
Instrumentation
All subjects were instrumented by the same operators and were supine on an electric motorized tilt table (Colin Medical Instruments Corp., San Antonio, TX) with a footboard. Beat-to-beat blood pressure was monitored using finger arterial plethysmography (Finometer; FMS, Amsterdam, The Netherlands), corrected for tilt angle and calibrated to brachial artery pressure. A single lead electrocardiogram measured HR. A nasal cannula connected to a capnograph with a pulse oximeter (Smiths Medical, Waukesha, WI) measured end tidal CO2 (ETCO2) and O2 saturation. TCD (Neurovision; Multigon, Yonkers, NY) measured CBFv of the left MCA using a 2 MHz probe fixed to the subject’s head by a custom-made headband. All analog signals were digitized at 200 Hz with custom signal processing software and analyzed off-line.
N-Back Task
A parametric N-back 23 using 2-Back levels presented the mental task. The visually presented stimulus duration was 1s, and inter-stimulus duration was 1s 13. Subjects responded to perceived correct 2-Back matches by pressing a button placed in their dominant hand. We used the number of correct responses to measure 2-back outcome.
Protocol
Subjects practiced responding, rested for five minutes, and then underwent three 2-back practice sessions. Baseline measurements of arterial pressure, CBFv, HR, and ETCO2 were taken during the last 5 minutes. Subjects rested for 15 minutes and then tilted upright to 15° for 10 minutes. The first minute of data were omitted to allow for HR and BP stabilization. Minutes 1-6 of the tilt were used to obtain mean and oscillatory data for that angle. The 2-back tasking started at minute 6 and lasted approximately 1 minute. After 10min, subjects were incrementally tilted to 30°, 45°, and 60°, and stabilization, baseline data collection, and 2-back repeated at each angle.
A priori stopping criteria during incremental tilt were signs and symptoms of presyncope; a decrease in systolic BP to 80 mmHg; a decrease in systolic BP to 90 mmHg with lightheadedness, nausea, sweating, or diaphoresis; or a request to discontinue testing. Presyncopal subjects were immediately returned to supine and testing ended. If subjects completed all angles of tilt they were returned to the supine position.
Functional Hyperemia
We used the change of CBFv (Δ(cm/s)/minute) as an index of functional hyperemia during 2-Back. This was quantitated by the slope of the CBFv during each 2-Back task at each angle of tilt as shown in Figure S1. CBFv varied from subject to subject in part because differences in the angle of insonation. Therefore, we normalized the CBFv slope to the average CBFv during measurement. Results are expressed as percent change in CBFv per minute. This quantity is positive for a net increase in CBFv (increased functional hyperemia) and negative for decreased functional hyperemia during mental activation.
Power Spectra and Transfer Function Analysis
Baseline and tilted MAP autospectra, mean CBFv autospectra, and transfer function analyses were obtained from data collected while supine and during minute 1-6 at each angle of tilt. Specific details of these calculations are shown in the online-only Data Supplement.
Data Analysis
All data were continuously sampled at 200 Hz, were converted with an analog-to-digital converter (DI-720 DataQ Ind, Milwaukee, WI) and analyzed offline. NCSS 2007 (NCSS, LCC, Kaysville, UT) statistical software was used in the analysis. Mean CBFv for each pulse was computed as a time average over a cardiac cycle. Analysis of 2-Back outcome and neuronal activation of CBFv (functional hyperemia) employed a repeated measures ANOVA conducted using one between factor (POTS vs. control) and one within factor (tilt angle at 5 pre-selected degrees). Data was mean ± standard error of the mean (SEM). Significance was set at P < 0.05.
Results
Supine – Baseline Data
Supine data are tabulated in Table 1. There was no significant difference in systolic blood pressure (SBP), diastolic blood pressure (DBP) or mean arterial blood pressure (MAP), ETCO2, or mean CBFv between POTS and controls. There was a significantly higher supine HR in POTS compared to controls (P<0.05).
Table 1.
Supine Hemodynamic Measurements
| Measurement | POTS | Control |
|---|---|---|
| SBP (mmHg) | 114 ± 3 | 111 ± 4 |
| DBP (mmHg) | 62± 2 | 61 ± 2 |
| MAP (mmHg) | 80 ± 2 | 78 ± 3 |
| HR (bpm) | 76 ± 5* | 65 ± 3 |
| ETCO2 (mmHg) | 41 ± 1 | 43 ± 1 |
| Mean CBFV (cm/sec) | 79 ± 3 | 76 ± 4 |
| OCBF power | 11.3 ± 1.8 | 14.9 ± 4.4 |
| OCBF: VLF power | 6.6 ± 1.2* | 10.1 ± 1.6 |
| OCBF: LF power | 3.4 ± 0.7 | 3.6 ± 1.3 |
| OCBF: HF power | 1.3 ± 0.2 | 1.2 ± 0.6 |
| OAP power | 10.6 ± 2.7 | 11.4 ± 3.0 |
| OAP: VLF power | 7.2 ± 3.1 | 8.0 ± 1.7 |
| OAP: LF power | 3.3 ± 0.8 | 2.8 ± 1.5 |
| OAP: HF power | 0.3 ± 0.1 | 0.6 ± 0.3 |
| 2-Back number correct | 6.9 ± 0.9 | 7.9 ± 0.2 |
| Functional Hyperemia (%change CBFv/min) | 4.1 ± 0.9 | 3.6 ± 1.2 |
= P<0.05 compared to control.
SBP =systolic BP, DBP=diastolic BP, MAP=mean arterial pressure, CBFv = cerebral blood flow velocity in the MCA, OCBF=oscillatory mean CBFv, OAP=oscillatory mean arterial pressure, VLF = very low frequency band, LF = low frequency band, HF= high frequency band, Units for OCBF is (cm/s)2. Units for OAP is (mmHg)2
Autospectral Power- Oscillatory Data
Supine autospectral data (OAP, and OCBF, equivalently MAP and mean CBFv variability data) are also shown in Table 1. VLF OCBF was significantly reduced in POTS compared to controls (P<0.01). There were no significant differences in total, LF and HF OCBF. There were no differences in oscillatory MAP power either total or divided amongst VLF, LF, and HF bands.
Transfer Function Analysis
Supine coherence, gain and phase are shown in Table 2. There was a lower gain in VLF in POTS compared to controls. Otherwise, there were no significant differences at any frequency band. Note that VLF coherence was always less than 0.5, implying either no relationship, a missing interacting term, a non-linear relationship, or the presence of excessive noise 24. Typically gain and phase data are often regarded as unreliable linear estimates under these circumstances.
Table 2.
MAP -> CBFv Transfer Function Analysis: Gain, Phase, and Coherence
| 0° | 15° | 30° | 45° | 60° | |
|---|---|---|---|---|---|
| OCBFv Power POTS | 11.3 ± 1.8 | 11.8 ± 1.3 | 14.4 ± 1.4 | 20.3 ± 1.7 | 26.4 ± 2.1*† |
| VLF | 6.6 ± 1.2 | 6.4 ± 1.6 | 5.8 ± 0.7 | 5.6 ± 1.0 | 5.3 ± 1.1 |
| LF | 3.4 ± 0.7 | 4.1 ± 0.9 | 7.6 ± 1.2 | 13.3 ± 1.3 | 18.7 ± 1.7*† |
| HF | 1.3 ± 0.2 | 1.3 ± 0.2 | 1.0± 0.1 | 1.4 ± 0.2 | 2.3 ± 0.7 |
| OCBFv Power Control | 16.9 ± 2.5 | 11.3 ± 1.9 | 11.2 ± 1.9 | 11.8 ± 1.9 | 12.5 ± 2.4 |
| VLF | 10.1 ± 1.6 | 6.5 ± 1.2 | 6.7 ± 2.2 | 6.4 ± 1.9 | 5.9 ± 1.9 |
| LF | 3.6 ± 1.3 | 3.4 ± 0.9 | 3.5 ± 1.0 | 4.1 ± 0.9 | 4.8 ± 1.1 |
| HF | 1.2 ± 0.6 | 1.4 ± 0.6 | 1.0 ± 0.3 | 1.3 ± 0.3 | 1.8 ± 0.5 |
| OAP Power POTS | 10.6 ± 2.7 | 13.9 ± 1.8 | 15.4 ± 1.3 | 17.7 ± 1.4 | 20.5 ± 2.3*† |
| VLF | 7.2 ± 3.1 | 8.3 ± 2.6 | 5.9 ± 1.0 | 3.0 ± 0.4 | 4.4 ± 1.4 |
| LF | 3.3 ± 0.8 | 4.6 ± 1.1 | 8.5 ± 1.2 | 13.4 ± 1.5 | 14.7 ± 1.2*† |
| HF | 0.3 ± 0.1 | 1.0 ± 0.1 | 1.0± 0.1 | 1.3 ± 0.2 | 1.4 ± 0.6 |
| OAP Power Control | 11.4 ± 3.0 | 11.3 ± 1.9 | 10.5 ± 1.9 | 11.7 ± 2.2 | 13.9 ± 2.4 |
| VLF | 8.0 ± 1.7 | 7.2 ± 1.9 | 6.3 ± 1.8 | 6.4 ± 1.9 | 7.2 ± 1.3 |
| LF | 2.8 ± 1.5 | 3.1 ± 0.4 | 3.4 ± 0.8 | 4.1 ± 0.8 | 6.2 ± 1.1 |
| HF | 0.6 ± 0.3 | 1.0 ± 0.2 | 0.9 ± 0.2 | 1.2 ± 0.2 | 1.5 ± 0.2 |
| Gain POTS | |||||
| VLF | 0.54±0.08 | 0.57±0.08 | 0.50±0.10 | 0.72 ± 0.13 | 0.50 ± 0.10 |
| LF | 0.80±0.11 | 0.85±0.06 | 0.87±0.07 | 1.0 ± 0.05 | 1.17 ± 0.07*† |
| HF | 1.00±0.12 | 1.04±0.07 | 0.93±0.10 | 0.90 ± 0.06 | 1.01 ± 0.08 |
| Gain Control | |||||
| VLF | 0.87 ± 0.12 | 0.66 ± 0.10 | 0.55 ± 0.07 | 0.58 ± 0.21 | 0.55 ± 0.12 |
| LF | 0.80 ± 0.11 | 0.86 ± 0.05 | 0.79 ± 0.09 | 0.72 ± 0.10 | 0.76 ± 0.06 |
| HF | 1.20 ± 0.16 | 1.07 ± 0.12 | 0.94 ± 0.08 | 0.90 ± 0.10 | 0.86 ± 0.09 |
| Phase POTS | |||||
| VLF | -58±19 | -29 ± 16 | -31 ± 12 | -46 ± 24 | -36 ± 15 |
| LF | -52±6 | -41 ± 6 | -36 ± 4 | -27 ± 4 | -18 ± 6*† |
| HF | -19±8 | -12 ± 6 | -17 ± 15 | -18 ± 4 | -21 ± 4 |
| Phase Control | |||||
| VLF | -51±11 | -68 ± 17 | -54 ± 11 | -40 ± 11 | -35 ± 8 |
| LF | -33±11 | -53 ± 7 | -49 ± 8 | -41 ± 3 | -40 ± 4 |
| HF | -9±6 | -9 ± 5 | -8 ± 4 | -19 ± 4 | -21 ± 4 |
| Coherence POTS | |||||
| VLF | 0.18 ± 0.05 | 0.42 ± 0.09 | 0.27 ± 0.07 | 0.27 ± 0.06 | 0.34 ± 0.03 |
| LF | 0.66 ± 0.06 | 0.79 ± 0.03 | 0.74 ± 0.03 | 0.89 ± 0.02 | 0.93 ± 0.03*† |
| HF | 0.81 ± 0.08 | 0.84 ± 0.03 | 0.85 ± 0.04 | 0.88 ± 0.02 | 0.76 ± 0.04 |
| Coherence Control | |||||
| VLF | 0.43 ± 0.06 | 0.49 ± 0.06 | 0.29 ± 0.08 | 0.34 ± 0.08 | 0.37 ± 0.08 |
| LF | 0.74 ± 0.07 | 0.68 ± 0.06 | 0.69 ± 0.08 | 0.73 ± 0.08 | 0.75 ± 0.09 |
| HF | 0.72 ± 0.09 | 0.80 ± 0.04 | 0.81 ± 0.08 | 0.76 ± 0.06 | 0.74 ± 0.10 |
= P<0.05 compared to 0° ;
= P<0.05 compared to control.
OCBFv = oscillatory mean cerebral blood flow velocity,
OAP=oscillatory mean arterial pressure,
VLF = very low frequency band; LF = low frequency band; HF= high frequency band.
Units for OCBFv are (cm/s)2 ; Units for OAP are (mmHg)2.
Units for gains are (cm•s-1•mmHg-1).
Units for phases is degrees. Coherence is dimensionless.
2-Back Results and Functional Hyperemia
There was no significant difference between POTS and controls in the number correct during 2-Back testing and in the functional hyperemic response associated with 2-Back testing while supine.
Imposition of Graded Orthostatic Challenge
Hemodynamic Data
Figure 1 shows that CBFv and systolic, diastolic and MAP changed similarly with angle of tilt for POTS and controls, while HR was increased in POTS and ETCO2 was somewhat decreased. Thus, while there were small significant differences observed in ETCO2, these did not result in decreased CBFv. CBFv was reduced (P<0.025) compared to supine in both POTS and controls but did not differ upright between groups.
Figure 1.
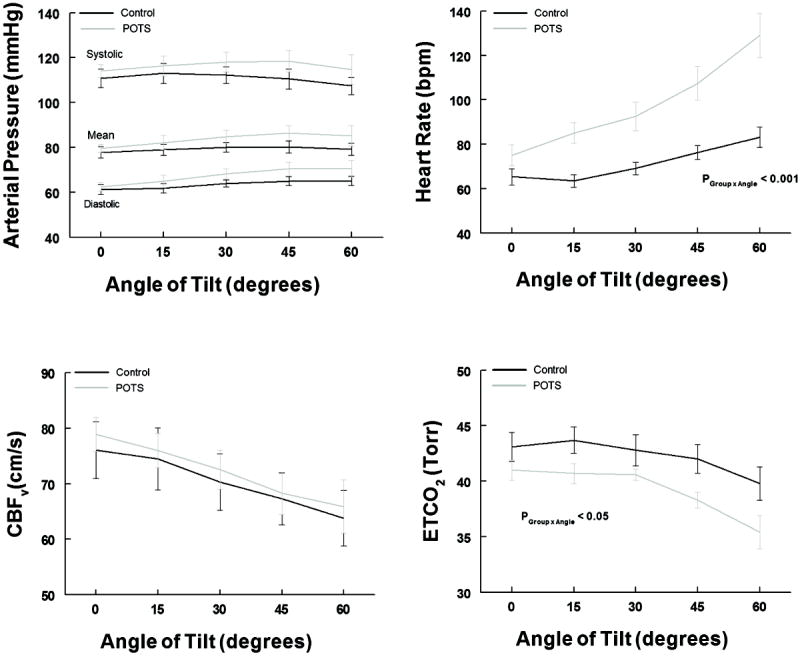
shows systolic, mean and diastolic arterial pressure in the left upper panel, Cerebral Blood Flow Velocity (CBFv) in the left lower panel, heart rate in beats per minute (bpm) in the right upper panel and End Tidal CO2 (ETCO2) in the right lower panel, all as a function of angle of tilt. Control is shown in black and POTS is in gray. There is no group difference in AP or CBFv. Heart rate is significantly increased in POTS (p<0.001 as shown) and ETCO2 is significantly decreased (p<0.05 as shown).
Autospectral Power- Oscillatory Data
Figure 2 shows data from a representative POTS and control subject. This illustrates the progressive increase in CBFv oscillations with angle of tilt in the POTS patient but not in the control subject.
Figure 2.
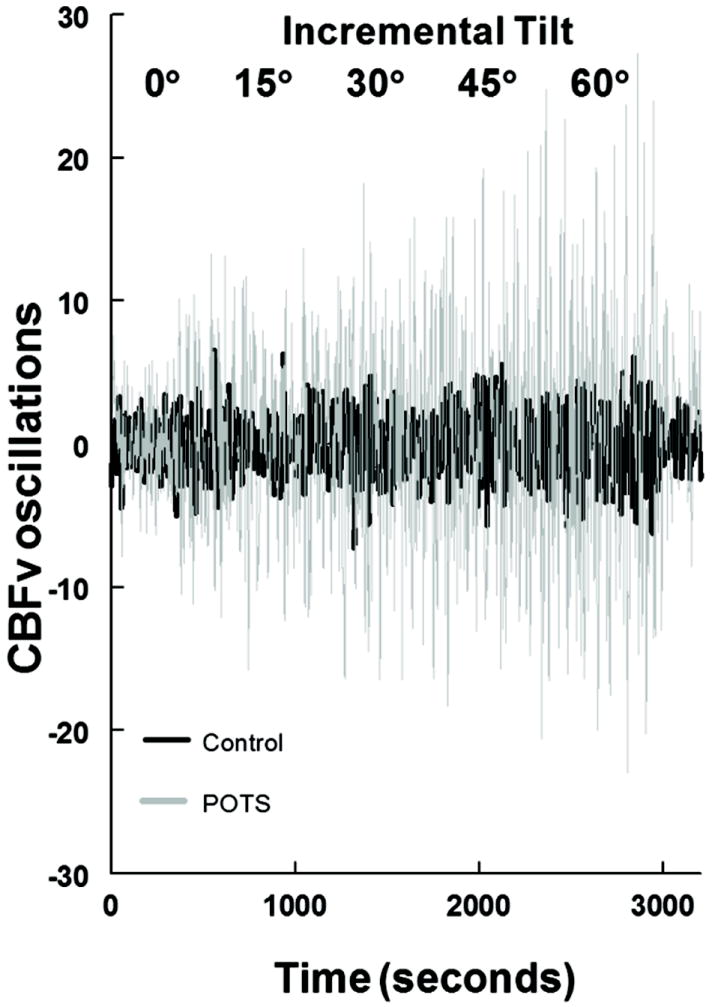
shows cerebral blood flow velocity (CBFv) oscillations during incremental tilt in a representative control subject (in black) and a representative POTS subject (in gray). Data were detrended as described in the text. Cerebral blood flow oscillations are progressively and markedly increased in POTS but more modestly increased in control.
Figure 3 shows the percent change in oscillatory mean CBFv and oscillatory MAP during incremental upright tilt. OAP and OCBF increase significantly (P<0.001) in POTS but not in controls. There are significant between group differences (P<0.001) which are more marked for OCBF than for OAP.
Figure 3.
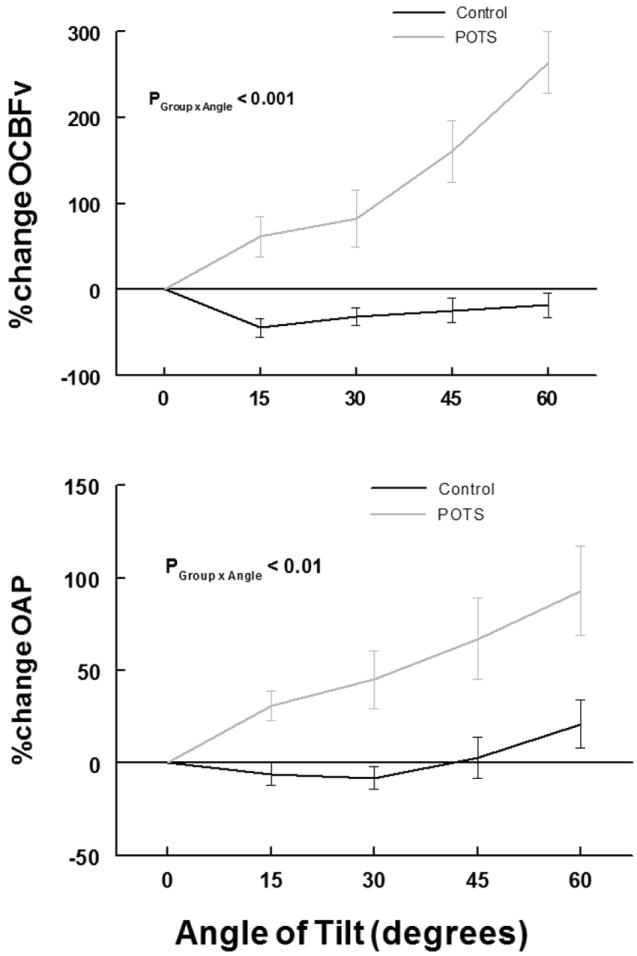
shows percent change in oscillatory cerebral blood flow velocity (%change OCBFv) (upper panel) and percent change in oscillatory arterial pressure (%change OAP) (lower panel), compared to pre-tilt values, averaged over all subjects within a group during incremental tilt. Control is in black and POTS is in gray. There are significant, large progressive increases in OCBFv (P < 0.001) and OAP (P < 0.01) with angle of tilt in POTS, but not control.
Table 2 shows data averaged over all subjects within each group. Significances within group and between groups are shown. Total oscillatory CBF velocity (OCBFv) and OAP power summed over all frequency bands increased progressively with angle of tilt in POTS (P< 0.001) but did not increase in control subjects. Table 2 also shows that the significant difference in total power between POTS and controls results predominantly from an increase in LF power in POTS not seen in control.
Transfer Function Analysis
Transfer function analysis data is also shown in Table 2. LF gain increases with angle of tilt in POTS (P<0.025) but not in controls, while VLF and HF gains were not different. Increasing gain was associated with increasing coherence and decreasing phase difference in POTS during incremental tilt.
2-Back Results and Functional Hyperemia
These data are depicted in Figure 4. There is a significant decrease in the accuracy of the 2-Back test results (P<0.01) and a significant reduction (P<0.001) in measured functional hyperemia in POTS, but not controls in whom 2-Back and functional hyperemia remained unchanged.
Figure 4.
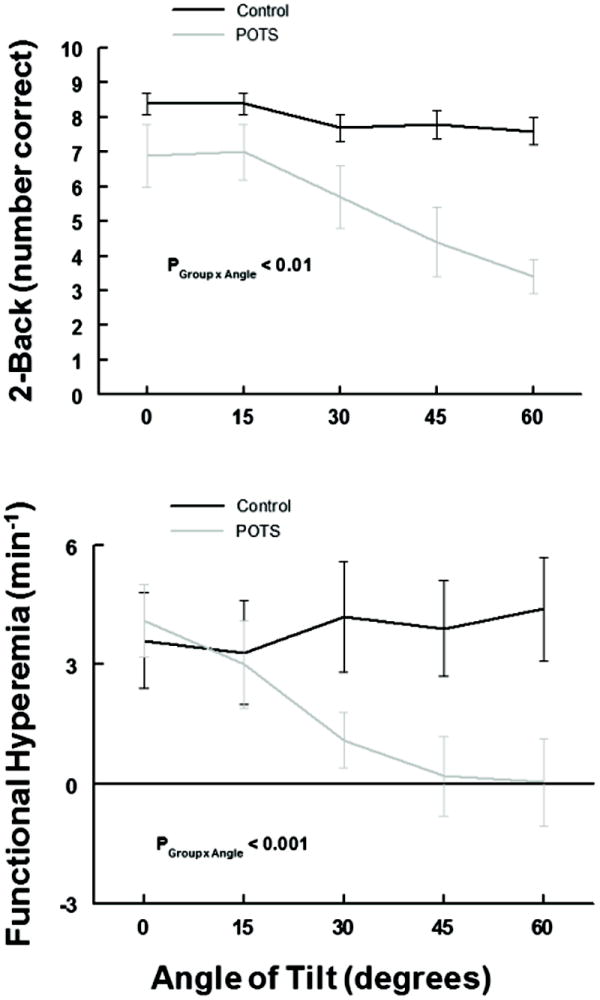
shows the number of correct answers during 2-Back testing (upper panel) and functional hyperemia (the rate of change of CBFv as Δ(cm/s)/minute) measurements (lower panel) averaged over all subjects within a group during incremental tilt. Control is in black and POTS is in gray. There are significant, large progressive decreases in 2-Back correct answers (P < 0.01) and in functional hyperemia (P < 0.001) in POTS, but not control.
Discussion
Our data show progressive reduction in 2-Back performance and functional hyperemia, and progressive increase in OCBF in POTS patients but not controls during step-wise incremental upright tilt. However, the average magnitude of CBFv decreases similarly in POTS and controls with angle of tilt. We observed stepwise increased oscillations in arterial pressure that produce larger stepwise increases in oscillatory CBF. Increased OCBF in POTS results primarily from enhanced LF oscillatory power that occurs in POTS, and not controls. This results from the combined effects of increased OAP in the LF band and increased LF transfer gain from OAP to OCBF in POTS. We have shown greatly increased CBFv in POTS when upright which is protected by an autoregulatory mechanism 18 which comprises properties of the vasculature that, in the absence of large environmental or metabolic changes, maintain CBF relatively unchanged despite changes in BP25. While the utility of these oscillations is poorly understood, our data suggest that OCBF interferes with NVC and that altered OCBF might serve as a marker for Brain Fog8 in POTS.
Progressively Increased OCBF implies Progressively Decreased Cerebral Autoregulation in POTS
The use of Fourier method based transfer function analysis is suitable for the evaluation of cerebral autoregulation (CA) in linear time-invariant systems 26 which are approximated during step-wise incremental tilt 12. CA is most effective at lower frequencies <=0.1 Hz, 27, therefore, OAP at frequencies corresponding to heart rate are transmitted to OCBF. However, they are highly damped at the tissue level with only LF and VLF oscillations effectively penetrating to the microvasculature28. While reduced coherence and increased phase difference between OAP and OCBF indicate relative independence of OAP and OCBF and thus very effective autoregulation, high gain, increased coherence, and decreased phase difference indicate ineffective autoregulation because of the great linear dependence of OCBF on OAP. Thus, in POTS but not controls, CA in the predominant LF band is progressively impaired as incremental tilt proceeds. This also implies that in POTS, at least, OCBF is driven by OAP at low frequency and is not predominantly the result of spontaneous vasomotion 29. Vasomotion may contribute to VLF oscillations between 0.01-.04Hz which are present in AP and CBF, and are the predominant oscillations in controls, and may reflect aspects of intact autonomous cerebrovascular myogenic regulation resulting in high CA since coherence is so poor.
Progressively Increased OCBF is Associated with Progressively Decreased Cognition and Neurovascular Coupling in POTS
Increasing OCBF power correlates fairly well with reductions in functional hyperemia and 2-Back performance. Mechanisms by which LF oscillations could perturb NVC during mental tasking may involve activation of astrocytic receptors resulting in arteriolar vasodilation 30-32. While neural activity controls local CBF via NVC, the “hemoneural hypothesis”33 proposes that local CBF reciprocally affects neuronal activity, i.e. a state of vasoneural coupling 34. OCBF could exert direct effects on neurons and axons, or indirect effects via astrocytes. Investigators 34-36 have demonstrated linkage between vascular stretch, astrocyte depolarization and release of vascular mediators37, promoting neuronal activity, and arachidonic acid metabolites. Oscillatory shear stress couples vascular deformation to astrocyte depolarization, and astrocyte depolarization to neuronal activity 38. Interference with NVC and neuronal depolarization may then result. Therefore, it is reasonable to infer that slow oscillations could interfere with NVC and that such interference could account for reduced cognitive performance in POTS.
Limitations
We show an associative rather than causal nature of the relationship between OCBF and functional hyperemia/neurovascular coupling and of 2-Back performance to NVC. While it is difficult to show statistical associations between physiological measurements and subjective phenomena, OCBF and Brain fog, we will need to devise experiments to show that altering OCBFv affects working memory. We do not know whether 2-Back task activated deficiencies in functional hyperemia in POTS during incremental tilt relate to reduced neural activity or to reduced neurovascular coupling; our techniques do not inform on neuronal activity.
TCD measures OCBFv rather than OCBF which depends on the cross-section area of the insonated artery. However, MCA cross-section may be relatively resistant to change during orthostatic stress 39. Also, oscillations of CBFv correspond to oscillations of CBF and to oscillations of MAP. Even under conditions of changing BP, CA can be estimated by TCD although the results may be a bit underestimated 40.
TCD only measures blood flow through specific cerebral blood vessels with good temporal resolution. The MCA was used because it is the main vessel that perfuses the brain area activated during working memory testing. While CBFv data represent an average over MCA perfused areas, perfusion during orthostatic stress may vary with brain location but such variations are often small. We did not measure TCD in both hemispheres as MCA CBFv was not different between hemispheres during orthostatic stress 15.
Fourier transfer function analysis depends on linear time-independent system characteristics. The linear hypothesis is an approximation but provides useful information. An additional drawback is the relatively small range of amplitudes of AP and CBFv that are interrogated, at least while supine and at lower angles of tilt. This improves with progressive increases in the tilt angle. Also, relatively small variations in BP occur in POTS.
Perspectives
Upright cognitive deficits are a defining feature of POTS, the mechanisms of which are unknown. It might be logical to hypothesize that a reduction in CBF flow would play some role as it does in neurogenic orthostatic hypotension and postural vasovagal syncope. Indeed, earlier work suggested that CBF was on average reduced in POTS compared to controls. Subsequent work did not show reduced mean CBF in most POTS patients leaving a gap in our understanding of potential pathophysiological origins of “Brain fog” in our patients. This study offers correlative data that may bridge that gap by showing associations among the deterioration of memory, neurovascular coupling, and increasing OCBF during progressive orthostatic stress. We hypothesize that causal vasoneural coupling exists alongside of neurovascular coupling and can result in malfunction of the neurovascular unit mediated by oscillatory blood flow. We speculate that our findings in POTS might generalize to other illnesses in which there is cognitive loss.
Supplementary Material
NOVELTY AND SIGNIFICANCE.
WHAT IS NEW
We show progressive reduction in neurocognitive performance and functional hyperemia, and a progressive increase in oscillatory cerebral blood flow in POTS patients but not controls during incremental upright tilt. This arises from a combination of increased oscillations in arterial pressure and reduced cerebral autoregulation in postural tachycardia syndrome.
WHAT IS RELEVANT
Increased oscillatory cerebral blood flow is thus associated with decreased cognition and decreased neurovascular coupling in postural tachycardia syndrome and may signify a causal relationship.
SUMMARY
Increased oscillatory cerebral blood flow is associated with reduced neurovascular coupling, diminished cognitive performance and deficient autoregulation in postural tachycardia syndrome. Altered oscillatory cerebral blood flow might serve as a marker for Brain Fog in postural tachycardia syndrome.
Acknowledgments
The authors would like to thank Ms. Seli Dzogbeta for her help in data collection and analysis.
Funding Sources
Funding for this project was provided by grants RO1 HL 112736 and RO1 HL 074873 from the National Heart Lung and Blood Institute
Footnotes
Disclosures
None
References
- 1.Robertson D. The epidemic of orthostatic tachycardia and orthostatic intolerance. Am J Med Sci. 1999;317:75–77. doi: 10.1097/00000441-199902000-00001. [DOI] [PubMed] [Google Scholar]
- 2.Stewart JM. Common syndromes of orthostatic intolerance. Pediatrics. 2013;131:968–980. doi: 10.1542/peds.2012-2610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Streeten DH, Anderson GH, Jr, Richardson R, Thomas FD. Abnormal orthostatic changes in blood pressure and heart rate in subjects with intact sympathetic nervous function: evidence for excessive venous pooling. J Lab Clin Med. 1988;111:326–335. [PubMed] [Google Scholar]
- 4.Streeten DH. Pathogenesis of hyperadrenergic orthostatic hypotension. Evidence of disordered venous innervation exclusively in the lower limbs. J Clin Invest. 1990;86:1582–1588. doi: 10.1172/JCI114878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schondorf R, Low PA. Idiopathic postural orthostatic tachycardia syndrome: an attenuated form of acute pandysautonomia? Neurology. 1993;43:132–137. doi: 10.1212/wnl.43.1_part_1.132. [DOI] [PubMed] [Google Scholar]
- 6.Low PA, Opfer-Gehrking TL, Textor SC, Benarroch EE, Shen WK, Schondorf R, Suarez GA, Rummans TA. Postural tachycardia syndrome (POTS) Neurology. 1995;45:S19–S25. [PubMed] [Google Scholar]
- 7.Jacob G, Shannon JR, Black B, Biaggioni I, Mosqueda-Garcia R, Robertson RM, Robertson D. Effects of volume loading and pressor agents in idiopathic orthostatic tachycardia. Circulation. 1997;96:575–580. doi: 10.1161/01.cir.96.2.575. [DOI] [PubMed] [Google Scholar]
- 8.Ross AJ, Medow MS, Rowe PC, Stewart JM. What is brain fog? An evaluation of the symptom in postural tachycardia syndrome. Clin Auton Res. 2013;23:305–311. doi: 10.1007/s10286-013-0212-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dewey RC, Pieper HP, Hunt WE. Experimental cerebral hemodynamics. Vasomotor tone, critical closing pressure, and vascular bed resistance. J Neurosurg. 1974;41:597–606. doi: 10.3171/jns.1974.41.5.0597. [DOI] [PubMed] [Google Scholar]
- 10.Ocon AJ, Medow MS, Taneja I, Clarke D, Stewart JM. Decreased upright cerebral blood flow and cerebral autoregulation in normocapnic postural tachycardia syndrome. Am J Physiol Heart Circ Physiol. 2009;297:H664–H673. doi: 10.1152/ajpheart.00138.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Del Pozzi AT, Schwartz CE, Tewari D, Medow MS, Stewart JM. Reduced cerebral blood flow with orthostasis precedes hypocapnic hyperpnea, sympathetic activation, and postural tachycardia syndrome. Hypertension. 2014;63:1302–1308. doi: 10.1161/HYPERTENSIONAHA.113.02824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stewart JM, Medow MS, Messer ZR, Baugham IL, Terilli C, Ocon AJ. Postural neurocognitive and neuronal activated cerebral blood flow deficits in young chronic fatigue syndrome patients with postural tachycardia syndrome. Am J Physiol Heart Circ Physiol. 2012;302:H1185–H1194. doi: 10.1152/ajpheart.00994.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Braver TS, Cohen JD, Nystrom LE, Jonides J, Smith EE, Noll DC. A parametric study of prefrontal cortex involvement in human working memory. Neuroimage. 1997;5:49–62. doi: 10.1006/nimg.1996.0247. [DOI] [PubMed] [Google Scholar]
- 14.Sabri O, Owega A, Schreckenberger M, Sturz L, Fimm B, Kunert P, Meyer PT, Sander D, Klingelhofer J. A truly simultaneous combination of functional transcranial Doppler sonography and H(2)(15)O PET adds fundamental new information on differences in cognitive activation between schizophrenics and healthy control subjects. J Nucl Med. 2003;44:671–681. [PubMed] [Google Scholar]
- 15.Ocon AJ, Messer ZR, Medow MS, Stewart JM. Increasing orthostatic stress impairs neurocognitive functioning in chronic fatigue syndrome with postural tachycardia syndrome. Clin Sci (Lond) 2012;122:227–238. doi: 10.1042/CS20110241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koehler RC, Roman RJ, Harder DR. Astrocytes and the regulation of cerebral blood flow. Trends Neurosci. 2009;32:160–169. doi: 10.1016/j.tins.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 17.Zhang N, Liu Z, He B, Chen W. Noninvasive study of neurovascular coupling during graded neuronal suppression. J Cereb Blood Flow Metab. 2008;28:280–290. doi: 10.1038/sj.jcbfm.9600531. [DOI] [PubMed] [Google Scholar]
- 18.Medow MS, Del Pozzi AT, Messer ZR, Terilli C, Stewart JM. Altered oscillatory cerebral blood flow velocity and autoregulation in postural tachycardia syndrome. Front Physiol. 2014;5:234. doi: 10.3389/fphys.2014.00234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lohmann H, Ringelstein EB, Knecht S. Functional transcranial Doppler sonography. Front Neurol Neurosci. 2006;21:251–260. doi: 10.1159/000092437. [DOI] [PubMed] [Google Scholar]
- 20.Panerai RB, Moody M, Eames PJ, Potter JF. Cerebral blood flow velocity during mental activation: interpretation with different models of the passive pressure-velocity relationship. J Appl Physiol (1985) 2005;99:2352–2362. doi: 10.1152/japplphysiol.00631.2005. [DOI] [PubMed] [Google Scholar]
- 21.Medow MS, Stewart JM. The postural tachycardia syndrome. Cardiol Rev. 2007;15:67–75. doi: 10.1097/01.crd.0000233768.68421.40. [DOI] [PubMed] [Google Scholar]
- 22.Raj SR. The Postural Tachycardia Syndrome (POTS): pathophysiology, diagnosis & management. Indian Pacing Electrophysiol J. 2006;6:84–99. [PMC free article] [PubMed] [Google Scholar]
- 23.Owen AM, McMillan KM, Laird AR, Bullmore E. N-back working memory paradigm: a meta-analysis of normative functional neuroimaging studies. Hum Brain Mapp. 2005;25:46–59. doi: 10.1002/hbm.20131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Taylor JA, Carr DL, Myers CW, Eckberg DL. Mechanisms underlying very-low-frequency RR-interval oscillations in humans. Circulation. 1998;98:547–555. doi: 10.1161/01.cir.98.6.547. [DOI] [PubMed] [Google Scholar]
- 25.Panerai RB. Assessment of cerebral pressure autoregulation in humans--a review of measurement methods. Physiol Meas. 1998;19:305–338. doi: 10.1088/0967-3334/19/3/001. [DOI] [PubMed] [Google Scholar]
- 26.Zhang R, Zuckerman JH, Giller CA, Levine BD. Transfer function analysis of dynamic cerebral autoregulation in humans. Am J Physiol. 1998;274:H233–H241. doi: 10.1152/ajpheart.1998.274.1.h233. [DOI] [PubMed] [Google Scholar]
- 27.Claassen JA, Levine BD, Zhang R. Dynamic cerebral autoregulation during repeated squat-stand maneuvers. J Appl Physiol (1985) 2009;106:153–160. doi: 10.1152/japplphysiol.90822.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li Z, Zhang M, Xin Q, Luo S, Cui R, Zhou W, Lu L. Age-related changes in spontaneous oscillations assessed by wavelet transform of cerebral oxygenation and arterial blood pressure signals. J Cereb Blood Flow Metab. 2013;33:692–699. doi: 10.1038/jcbfm.2013.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Haddock RE, Hill CE. Rhythmicity in arterial smooth muscle. J Physiol. 2005;566:645–656. doi: 10.1113/jphysiol.2005.086405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zonta M, Angulo MC, Gobbo S, Rosengarten B, Hossmann KA, Pozzan T, Carmignoto G. Neuron-to-astrocyte signaling is central to the dynamic control of brain microcirculation. Nat Neurosci. 2003;6:43–50. doi: 10.1038/nn980. [DOI] [PubMed] [Google Scholar]
- 31.Koehler RC, Gebremedhin D, Harder DR. Role of astrocytes in cerebrovascular regulation. J Appl Physiol (1985) 2006;100:307–317. doi: 10.1152/japplphysiol.00938.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Filosa JA, Iddings JA. Astrocyte regulation of cerebral vascular tone. Am J Physiol Heart Circ Physiol. 2013;305:H609–H619. doi: 10.1152/ajpheart.00359.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moore CI, Cao R. The hemo-neural hypothesis: on the role of blood flow in information processing. J Neurophysiol. 2008;99:2035–2047. doi: 10.1152/jn.01366.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.White RP, Hindley C, Bloomfield PM, Cunningham VJ, Vallance P, Brooks DJ, Markus HS. The effect of the nitric oxide synthase inhibitor L-NMMA on basal CBF and vasoneuronal coupling in man: a PET study. J Cereb Blood Flow Metab. 1999;19:673–678. doi: 10.1097/00004647-199906000-00011. [DOI] [PubMed] [Google Scholar]
- 35.Witthoft A, Em KG. A bidirectional model for communication in the neurovascular unit. J Theor Biol. 2012;311:80–93. doi: 10.1016/j.jtbi.2012.07.014. [DOI] [PubMed] [Google Scholar]
- 36.Cao R. The hemo-Neural Hypothesis: Effects of Vasodilation on Astrocytes in the Mammalian Neocortex. Masssachusetts Institute of Technology; Feb 20, 0011. [Google Scholar]
- 37.Takabe W, Jen N, Ai L, Hamilton R, Wang S, Holmes K, Dharbandi F, Khalsa B, Bressler S, Barr ML, Li R, Hsiai TK. Oscillatory shear stress induces mitochondrial superoxide production: implication of NADPH oxidase and c-Jun NH2-terminal kinase signaling. Antioxid Redox Signal. 2011;15:1379–1388. doi: 10.1089/ars.2010.3645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kozlov AS, Angulo MC, Audinat E, Charpak S. Target cell-specific modulation of neuronal activity by astrocytes. Proc Natl Acad Sci U S A. 2006;103:10058–10063. doi: 10.1073/pnas.0603741103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Serrador JM, Picot PA, Rutt BK, Shoemaker JK, Bondar RL. MRI measures of middle cerebral artery diameter in conscious humans during simulated orthostasis. Stroke. 2000;31:1672–1678. doi: 10.1161/01.str.31.7.1672. [DOI] [PubMed] [Google Scholar]
- 40.Liu J, Zhu YS, Hill C, Armstrong K, Tarumi T, Hodics T, Hynan LS, Zhang R. Cerebral autoregulation of blood velocity and volumetric flow during steady-state changes in arterial pressure. Hypertension. 2013;62:973–979. doi: 10.1161/HYPERTENSIONAHA.113.01867. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


