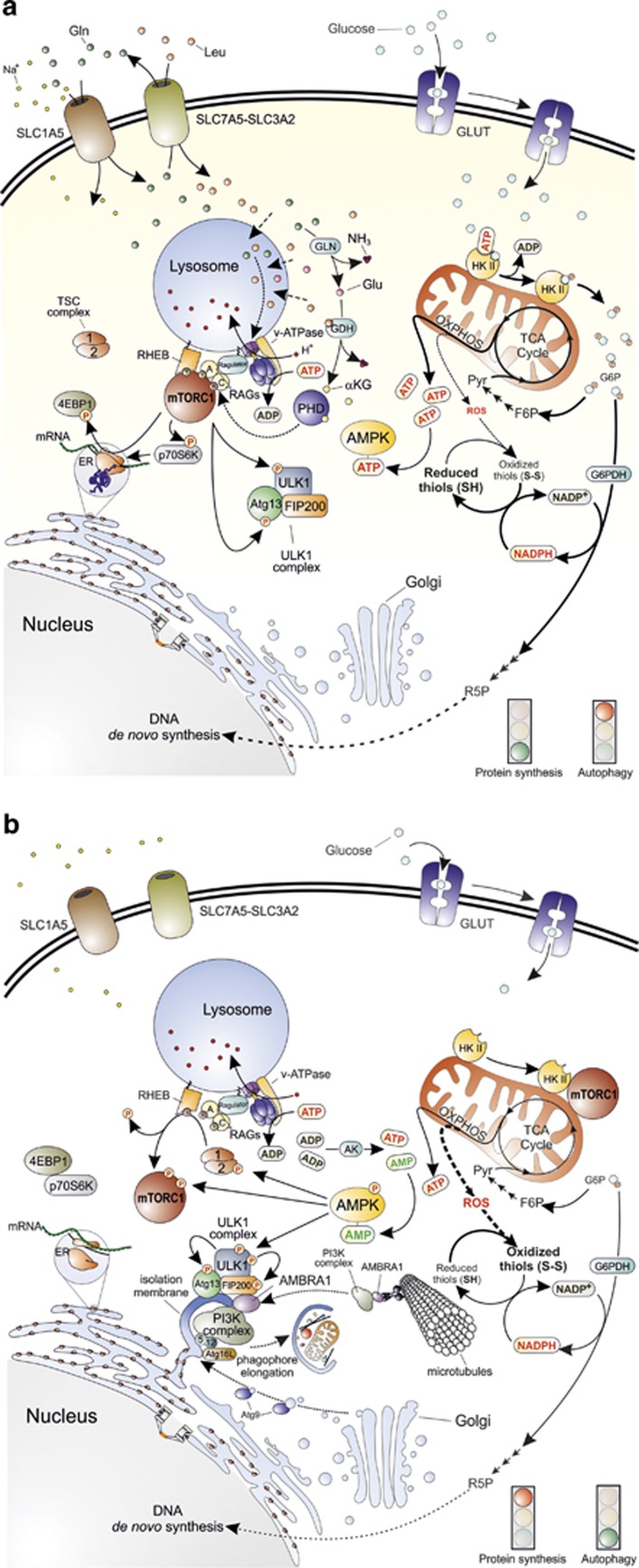
Figure 1.
Main molecular pathways activated in the presence or absence of nutrients. (a) The synergic import of leucine (Leu) and glutamine (Gln) (top left) results in mTORC1 recruitment to the lysosomal membrane and its subsequent activation by at least two distinct pathways. The first one proposes that cytosolic amino acids enter the lysosome and signal their presence to RAG-A and RAG-C (or RAG-B and RAG-D, not shown in the figure) through the lysosome-located proton pump v-ATPase and the multimolecular complex called Ragulator. The second pathway provides for the double deamination of Gln catalysed by the enzymes glutaminase (GLN) and glutamate dehydrogenase (GDH). This sequence of reactions subsequently generates glutamate (Glu) and α-ketoglutarate (αKG) that, by acting as co-substrate for prolyl hydroxylases (PHD), finally leads to RAG activation. GTP-bound RAG-A (or B) and GDP-bound RAG-C (or D) can then recruit mTORC1 to the lysosome membrane where it is activated by RHEB (middle left). Once activated, mTORC1 activates protein synthesis by phosphorylating 4EBP1 and p70S6K, and concomitantly inhibits autophagy by phosphorylating ULK1 complex at the level of ULK1 and Atg13 (bottom left). Glucose is taken up through specific transporters (GLUTs) and phosphorylated to glucose-6-phosphate (G6P) by hexokinase (the only mitochondrial isoform II, HKII, is shown in the top right side of the figure). G6P is then isomerized to fructose-6-phosphate (F6P), oxidized through the glycolytic pathway to generate pyruvate (Pyr) and acetyl-CoA that fuels the mitochondrial TCA cycle and the respiratory chain for the production of ATP (middle right) through the oxidative phosphorylation (OXPHOS). G6P can also undergo oxidation via the glucose-6-phosphate dehydrogenase (G6PDH)-mediated catalysis along the pentose phosphate pathway (middle right). In this way, electrons required for NADP+-to-NADPH reduction, and sugars needed for DNA de novo synthesis (e.g., ribulose-5-phosphate, R5P) are also provided (bottom right). (b) Upon amino acid deprivation, RAGs exchange nucleotides located in their binding sites (GTP with GDP or vice versa), thus leading to mTORC1 release from the lysosome membrane (top left). These 2 events are associated with the inhibitory binding of mTORC1 to HKII that takes place upon glucose deficiency and G6P level decrease (top right). This condition leads to a decrease of NADPH and ATP levels that finally result in a reduced antioxidant capacity of the cell (especially in regenerating the reduced thiol pool) (middle right) and in energetic stress that the cell attempts to counteract by the adenylate kinase (AK)-mediated conversion of ADP into ATP and AMP (centre). AMP increase induces to the activation of AMPK that inhibits protein synthesis by phosphorylating TSC2 and mTORC1 and activates autophagy by phospho-activating ULK1. Once activated, ULK1 phosphorylates its interactors (Atg13 and FIP200) and recruits microtubule-associated PI3K complex by means of an AMBRA1-mediated process to initiate the nucleation phase of autophagic vesicles from the endoplasmic reticulum (or mitochondria, not shown). Many other factors, such as Atg proteins coming from Golgi apparatus (e.g., Atg9) contribute to phagophore elongation and autophagosome formation (bottom)