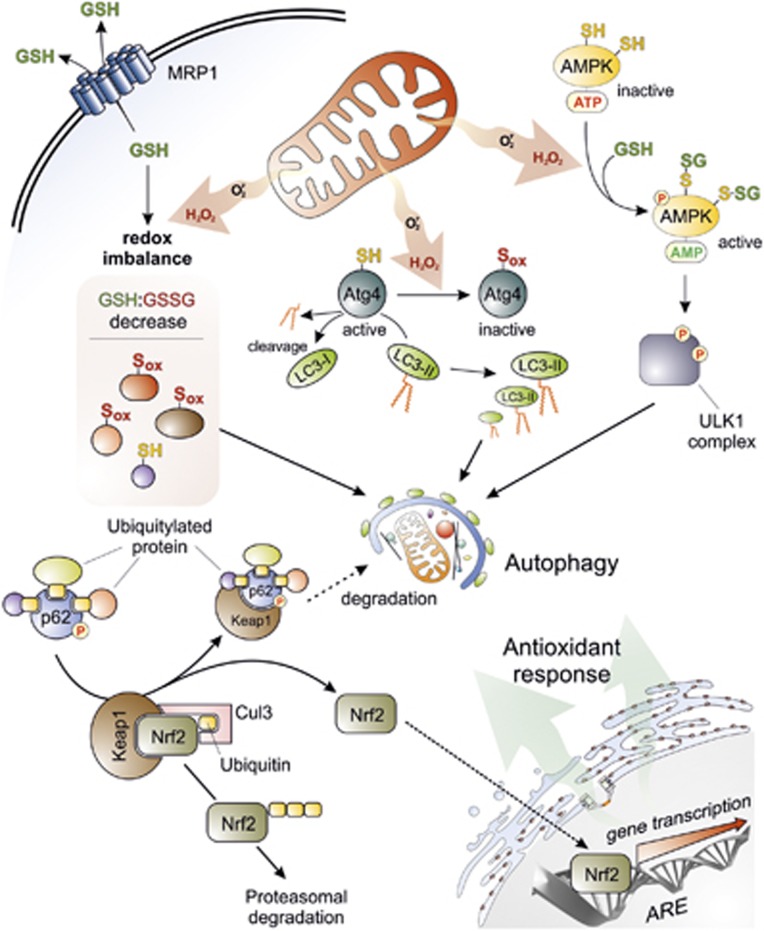
Figure 2.
Crosstalk between autophagy and oxidative stress. Superoxide (O ) and H2O2 are the main ROS produced by mitochondria upon nutrient deprivation. They positively regulate autophagy by means of at least three different mechanisms, including: (1) S-glutathionylation (SH → S-SG) of cysteines located in the α and β subunits of AMPK (top right); (2) oxidation of Cys81 (SH → Sox) of Atg4 that in turn leads to the inactivation of its ‘delipidating' activity on LC3 and to the accumulation of the pro-autophagic LC3-II isoform (top centre); and (3) wide alteration of thiol redox state (e.g., decrease of GSH/GSSG ratio and general increase of oxidized thiols, Sox) that is facilitated by the release of reduced glutathione (GSH) to the extracellular milieu through the multidrug resistance protein 1 (MRP1) (top left). In a redox-independent manner, it has also been demonstrated that p62, when bound to ubiquitylated protein aggregates, can undergo phosphorylation on Ser351, thereby sequestering Keap1 and leading to its detachment from Nrf2 (bottom left). Consequently, Nrf2 is no longer degraded by the ubiquitin-3 proteasome system, but translocates in the nucleus, binds to antioxidant-responsive elements (AREs) located in the promoter regions of antioxidant genes and activates their transcription (bottom right)
) and H2O2 are the main ROS produced by mitochondria upon nutrient deprivation. They positively regulate autophagy by means of at least three different mechanisms, including: (1) S-glutathionylation (SH → S-SG) of cysteines located in the α and β subunits of AMPK (top right); (2) oxidation of Cys81 (SH → Sox) of Atg4 that in turn leads to the inactivation of its ‘delipidating' activity on LC3 and to the accumulation of the pro-autophagic LC3-II isoform (top centre); and (3) wide alteration of thiol redox state (e.g., decrease of GSH/GSSG ratio and general increase of oxidized thiols, Sox) that is facilitated by the release of reduced glutathione (GSH) to the extracellular milieu through the multidrug resistance protein 1 (MRP1) (top left). In a redox-independent manner, it has also been demonstrated that p62, when bound to ubiquitylated protein aggregates, can undergo phosphorylation on Ser351, thereby sequestering Keap1 and leading to its detachment from Nrf2 (bottom left). Consequently, Nrf2 is no longer degraded by the ubiquitin-3 proteasome system, but translocates in the nucleus, binds to antioxidant-responsive elements (AREs) located in the promoter regions of antioxidant genes and activates their transcription (bottom right)