Abstract
Recent work involving intracranial recording during human memory performance provides superb spatiotemporal resolution on mnemonic processes. These data demonstrate that the cortical regions identified in neuroimaging studies of memory fall into temporally distinct networks and the hippocampal theta activity reported in animal memory literature also plays a central role in human memory. Memory is linked to activity at multiple interacting frequencies, ranging from 1-500 Hz. High-frequency responses and coupling between different frequencies suggest that frontal cortex activity is critical to human memory processes, as well as a potential key role for the thalamus in neocortical oscillations. Future research will inform unresolved questions in the neuroscience of human memory and guide creation of stimulation protocols to facilitate function in the damaged brain.
Introduction
Our ability to act based on personal experience drawn from memory is central to everyday life, and defines our individual identity. Human memory function is susceptible to a wide range of neurological insults. For instance, dementia and associated memory dysfunction are reaching epidemic levels as our population ages [1]. We must understand the precise neural mechanisms governing memory to make inroads into the understanding of normal as well as disordered memory. Subdural and depth recordings – termed electrocorticography (ECoG) or intracranial electroencephalography (icEEG) – offer superb temporal and spatial resolution that is unparalleled in the study of human cognition. The present review focuses on the contributions of subdural and depth recordings obtained during the successful encoding and immediate or delayed retrieval of memories in humans. We argue that ECoG/icEEG informs unresolved questions in the study of human memory and is yielding insights necessary for the development of novel interventions to facilitate memory function in the damaged brain. We will use the term ECoG to subsume both subdural (epicortical) and depth (subcortical) recordings.
It is well-known that the hippocampus and surrounding medial temporal lobe (MTL) structures are necessary for episodic memory (long-term memory for personal events) [1,2], and that lateral prefrontal cortex (PFC) is necessary for working memory (active storage and processing in memory) [3,4]. However, working and episodic memory, although often approached as separate topics in psychology, both depend on MTL-PFC interactions. What is the nature of these and other inter-regional interactions, and might they be fractionated depending on the type of memory in question? Furthermore, how do neural networks differentially support encoding and retrieval operations, even within a given type of memory? Might the PFC play a domain-general role in memory – i.e., a global role that is not specific to stimulus modality or encoding or retrieval operation – that is comparable and/or complimentary to the role of the MTL?
Lesion studies and functional magnetic resonance imaging (fMRI) reveal the where of memory function. Scalp EEG and magnetoencephalography (MEG) reveal the when and, for low-frequency spectral activity and event-related potentials (ERPs), the how of memory. In contrast, ECoG has superb spatiotemporal resolution and can measure an expanded frequency range of activity, including high-frequency responses and, in rare instances, single-unit activity (SUA). Thus, ECoG can reveal the how of human memory across an extended scope of the neurophysiology of memory in humans. For instance, Burke and colleagues [5] reported the results of a large-scale ECoG study of subsequent memory (SM) – i.e., measures of neural activity correlated with or predictive of later remembering (see [6]). Their data reveal that regions previously identified using fMRI fall into two networks that exhibit spatiotemporally distinct patterns of 64-95 Hz gamma band power activity within the first 1.5 sec following encoding of each later recalled word – first in the ventral visual pathway and MTL, and then across association regions including left-lateralized inferior frontal, posterior parietal, and ventrolateral temporal cortices. The authors suggest that these networks reflect higher-order visual processes followed by top-down control mechanisms [5]. Jacobs et al. [7] used MTL depth recordings to reveal that neuronal firing is phase-locked to oscillatory activity in the delta, theta, and gamma frequency bands in humans. Comparable invasive recording has been traditionally restricted to animals; thus, human ECoG recording provides a powerful bridge to the animal literature on memory processing.
Event-related potentials and medial temporal lobe function
Intracranial recordings provide the spatial resolution needed to explore temporal dynamics of memory within subregions of the MTL. Axmacher and colleagues [8] examined SM using ECoG and fMRI, and found that, unlike words that were successfully recalled, words that were later forgotten deactivate the hippocampus at encoding – manifested by a positive direct current response in ECoG and negative blood-oxygen-level dependent (BOLD) response in fMRI. This effect was apparent for words presented both early and late in a list, suggesting a continuum of hippocampal involvement over long and short retention durations. Other studies of encoding as a function of subsequent recall demonstrate that the successful encoding of words is associated with an early (300-400 msec) negative ERP in the rhinal cortex, followed by a late (500 msec or later) positive hippocampal ERP [9,10]. Viewing encoding as a function of subsequent recognition, as opposed to recall, several studies suggest that SM is linked to negative ERPs in the hippocampus. In preparation for immediate recognition, a negative ERP is observed 300-500 msec after stimulus presentation and again upon presentation of the probe at retrieval [11]. In preparation for delayed recognition, a late hippocampal negative ERP is observed following stimulus presentation [12-14]. The differential ERP effects observed in hippocampal activity depending on whether SM is measured by recall or recognition suggest that the hippocampus plays a selective role in recollection (i.e., specific memory, in contrast to strength-based familiarity; see [15]). However, additional data indicate that there is more to the human MTL story than ERPs. For instance, negative-polarity ERPs have been shown to correlate with high-gamma activity [14] (see section on high-frequency responses, below).
Intracranial recordings have also informed the long-standing debate over the difference between recollection and familiarity [15]. Staresina et al. [16] demonstrated that the perirhinal cortex and hippocampus are qualitatively dissociable at retrieval, revealing that the magnitude of the ERP in each MTL subregion region differs between the successful recognition of an item versus a source detail (e.g., background color), versus correct rejection of an item. The hippocampus shows enhanced ERP activity during the retrieval of source information as compared to item retrieval or correct rejection [16]. Rutishauser et al. [17] used microelectrodes to record activity of single neurons (SUA) in the hippocampus and amygdala. Encoding is associated with sustained local SUA – with the highest spike rate observed during encoding of items later recollected (here, retrieved with correct source information), followed by items considered familiar (retrieved without source information), and the lowest SUA rate observed for items not recognized – supporting a continuous strength model of retrieval. Together, the high spatiotemporal resolution of ECoG provides evidence supporting both a continuous strength model of hippocampal function [16,17] and a dual-process model of retrieval by MTL subregion [16].
Low-frequency responses and memory
The animal literature has provided robust evidence of oscillations in the theta frequency band (3-8 Hz) at encoding in MTL structures (e.g., [18]). In humans, direct intra-MTL depth recordings and/or cortical surface recordings have shown that presentation of a subsequently remembered stimulus resets theta activity or alters theta power in the hippocampus, rhinal cortex, and/or amygdala [10,12-14,19,20], as well as temporal, frontal, and/or parietal-occipital association cortices [21-24]. Shifts in other frequency bands tend to co-occur with these shifts in theta. Specifically, theta and alpha phase resets in temporal, parietal, and occipital cortices [21]; theta, alpha, and beta phase resets [12] or power decreases [20] in the MTL; and right cortical theta power increases in the midst of widespread gamma increases [25] have been linked to SM. Employing SUA measurements, Rutishauser et al. [19] reported that theta phase resets are tightly coupled with local spiking activity – i.e., theta phase-SUA coupling (see [26]). Critically, hippocampal theta phase-SUA coupling predicts subsequent long-term recognition as well as participants’ confidence in their responses at retrieval. Suthana and colleagues [27] found that stimulation of the entorhinal cortex during encoding resets the theta phase in the hippocampus and enhances spatial memory, suggesting a causal role for hippocampal theta activity in SM.
Patterns of theta and successive alpha band power increases in the hippocampus and rhinal cortex just prior to stimulus presentation have also been shown to predict subsequent recognition [28] (Figure 1). Fell et al. [28] proposed that this pattern reflects the coupling of activated contextual information (theta) and top-down control processes (alpha). This intracranial finding demonstrates the importance of preparatory membrane excitability in successful encoding.
Figure 1.
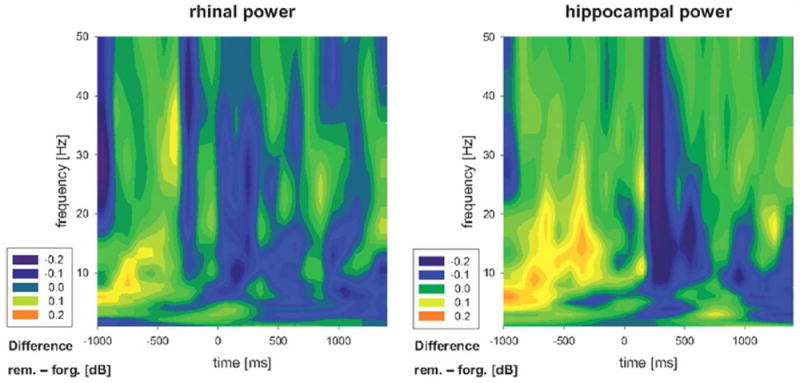
Normalized power difference plots for the contrast of subsequently remembered > forgotten words in the rhinal cortex (left) and hippocampus (right) reported in [28]. Power increases are shown primarily in the theta and alpha bands prior to stimulus presentation at encoding. Adapted from [28].
There is activity in multiple frequencies occurring simultaneously during memory encoding and retrieval. Successful encoding in humans has been linked to two distinct hippocampal oscillations at the edges of the theta band. Lega et al. [29] found that the ‘slowtheta’ exhibits higher power at stimulus presentation and is selectively coupled with power in the gamma frequency band. (See Box 1 for a description of interactions between different frequency bands, i.e., cross-frequency coupling.) This pattern is also observed just prior to recall; taken together, Lega et al. [29] argue that this 3 Hz activity may be the human analogue of the theta oscillations observed in animals. At retrieval, Watrous et al. [30] showed that coherence between MTL, PFC, and parietal cortex is increased for correctly retrieved source information. Spatial memory is linked to 1-4 Hz oscillations while temporal memory is linked to 7-10 Hz oscillations in these regions, supporting a multiplexing mechanism wherein different frequency bands support distinct memory operations (Figure 2). Although multiplexing is not specific to memory, these results suggest that the phenomenon of frequency multiplexing within and across regions may be central to human memory capacity [31].
Box 1. Additional elements.
Phase-amplitude coupling in memory
Cross-frequency coupling refers to interactions between different frequency bands; it can refer to the coupling of different phases or amplitudes, or the phase of one frequency and the amplitude of another (i.e., phase-amplitude coupling). Coupling between higher-frequency amplitude and lower-frequency phase has been linked with a variety of cognitive and motor functions [62-64], and proposed as a mechanism for the short-range coding and inter-regional communication and integration of information [51,52]. Axmacher and colleagues [44] reported that successful maintenance of multiple items in working memory is associated with enhanced coupling between theta phase and beta and low-gamma (14-50 Hz, peak at 28 Hz) amplitudes in the hippocampus (also [46]). Furthermore, memory load modulates theta phase to incorporate additional envelopes of higher-frequency activity, with theta peaking at 7 Hz. At retrieval, Foster et al. [33] reported that recognition of remote autobiographical memories correlates with enhanced coupling between theta phase and high-frequency amplitude. The magnitude of phase-locking between the hippocampus and retrosplenial cortex in the theta band peaks 300-400 msec prior to high-frequency (70-180 Hz) peak amplitude.
Phase-amplitude coupling also supports communication pathways between the thalamus and frontal cortex during successful memory encoding and retrieval. Staudigl et al. [49] revealed that successful retrieval is linked to thalamus-frontal synchrony and enhanced coupling between inter-regional beta phase and 55-80 Hz gamma amplitude. Furthermore, they found that the beta activity modulated patterns of gamma power.
Figure 2.
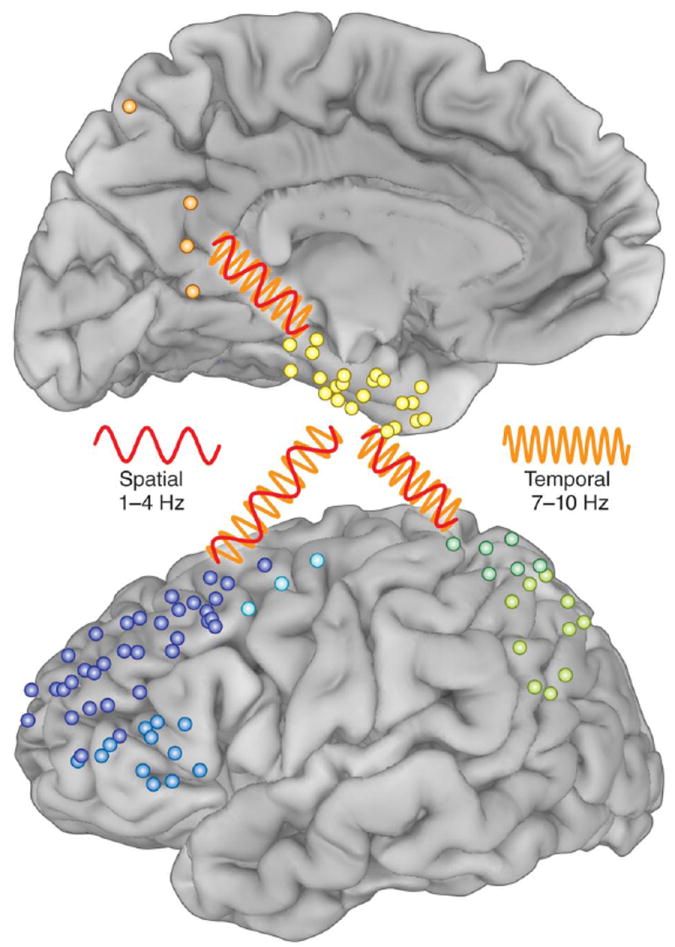
Individual subdural recording sites from the patients studied in [30]; blue, prefrontal; green, parietal; orange, precuneus; yellow, parahippocampal. The red oscillation (1-4 Hz) represents coherence between brain regions during spatial memory and the orange oscillation (7- 10 Hz) represents coherence between these regions during temporal memory. Adapted from [30,31].
Evidence from multiple studies indicates that theta is involved in timing coordinated activity within and across regions during successful encoding and retrieval. Within the hippocampus, theta power first increases and later decreases following presentation of stimuli that are subsequently recognized [14]. Fell et al. [13] showed that synchrony between the hippocampus and rhinal cortex varies as a function of frequency, with increased synchrony in delta and theta bands, but increased followed by decreased synchrony in the low-gamma band (28-46 Hz); phase-locking within each region also varies over time as a function of frequency. Using both subdural and depth recordings to examine network activity, Burke et al. [24] showed that theta and gamma power increase in a posterior-to-anterior direction with time over widespread cortical as well as hippocampal regions. Importantly, while some spectral modulations co-occur with local inter-regional synchrony and others with local or global asynchrony, synchronous activity for verbal SM is hubbed in the left PFC. An outstanding question in the intracranial recording of memory concerns whether the PFC may serve a domaingeneral, causal role as part of an MTL-PFC network, or whether the role of neocortical regions is dictated by domain-specific representations at encoding and/or cognitive operations at retrieval.
Anderson and colleagues [32] showed that increased theta power in the MTL precedes increased theta power in the PFC during successful recall, and serves to synchronize the two regions. Two studies reported that retrieval of remote autobiographical memories (i.e., memories encoded prior to entering the laboratory) is associated with theta band coherence between the MTL and other regions – such as phase-locking between the MTL and retrosplenial cortex [33]. Steinvorth et al. [34] showed that layers of the entorhinal cortex that project outward to the cortex exhibit theta activity during retrieval that is synchronized with theta activity in the frontal and temporal cortices. Layers that project inward to the hippocampus, however, show increased gamma activity. Taken together, these findings suggest that theta is important in long-range communication during successful retrieval.
High-frequency responses and memory
Activity in gamma and higher-frequency bands (50-500 Hz) is important for representing information within neural regions. Notably, activity in frequency ranges above 70-80 Hz correlates with local spiking activity [35,36], suggesting that these high-frequency responses reflect SUA. High-frequency power increases [37] and oscillatory activity [38] have been shown to represent individual stimuli in the neocortex, and gamma band activity is sensitive to differences between stimuli [39]. High-frequency signals are less than a microvolt in amplitude at the scalp, placing them in the noise range of scalp EEG recording. Thus, reliable data in highfrequencies is generally limited to subdural or depth recording (cf. [22]). Importantly, ECoG studies demonstrate that low- and high-frequency activity often share an inverse relationship [5,20,24,25,38,39,40]. Likewise, while measures of high-frequency amplitude show overlap with the BOLD measures of fMRI, low-frequency activity is anatomically dissociated with BOLD measures [41].
Intracranial recordings reveal an important role for high-frequency responses in successful episodic memory encoding and retrieval. Sederberg and colleagues [42] demonstrated that SM is linked to power increases in the 28-100 Hz gamma range in subdural and depth electrodes in the hippocampus, temporal cortex, and PFC at encoding (also [5,20,22]), and that this pattern is reinstated just prior to correctly recalling words. Kucewicz et al. [38] reported that encoding images induces oscillations from 50-500 Hz within the primary visual cortex as well as limbic and higher cortical regions, consistent with the visual processing stream (also [5]), and successful recall is linked with increased 50-500 Hz oscillatory activity in widespread higher cortical regions. Within the hippocampus, Park et al. [40] revealed a role for high-gamma (51-100 Hz) but not low-gamma, delta, or theta, in successful encoding during navigation. Axmacher et al. [14] found that 70-90 Hz high-gamma power is selectively increased during processing of unexpected items at multiple points during encoding in preparation for a recognition test. In addition to subserving encoding in conjunction with synchronization and desynchronization in the theta band, this high-gamma activity is also correlated with the hippocampal N500 ERP. Foster et al. [43] showed that 70-180 Hz power peaks in the hippocampus-connected posteromedial cortex after 400 msec during retrieval of autobiographical memories.
Gamma and higher-frequency band power responses have also been used to study working memory, suggesting a complex picture of working memory function that involves oscillations and sequenced spiking activity. Axmacher et al. [44] demonstrated that maintenance of multiple items in working memory is linked to the coupling of neural assemblies in the 25-100 Hz gamma range with theta phase in the hippocampus. Furthermore, increasing the number of items (i.e., memory load), is associated with modulating this cross-frequency coupling by increasing the length of theta cycles to incorporate additional pockets of higher-frequency power increases (Box 1). Indeed, Roux and Uhlhaas [45] argued that ‘theta-gamma’ (gamma range: 30-200 Hz) coupling subserves the organization of items maintained in sequence (also [46]), while alpha-gamma coupling may also support the active inhibition of task-irrelevant processes integral to complex working memory.
Inter-regional coherence and memory
Memory is supported by both the local connectivity of MTL subregions, and the distributed connectivity of the MTL to other cortical and subcortical regions [1]. Intracranial recordings can provide insight into the dynamics of both short- and long-range communication. Fell and colleagues reported that successful encoding is associated with hippocampus-rhinal cortex coherence in the delta and theta bands [13,47] and, in the gamma band, with early hippocampus-rhinal cortex synchronization and later desynchronization [13,48] (also [49]). Comparable patterns of within-theta and within-gamma bands also occur over left cortical regions [24]. Synchronization within theta and gamma bands is both multiplexed and dissociable throughout the MTL and cortex in SM [24]. At retrieval, multiplexing occurs as a function of the source being retrieved – with spatial and temporal information traveling along different frequency bands but within comparable MTL-PFC-parietal networks (Figure 2) [30,31]. Anderson et al. [32] suggested that one mechanism of coherence between the MTL and PFC is supported by synchronous activity in the theta band during successful recall.
Intracranial recordings of human cognition also suggest how communication might occur between synchronized regions. Staudigl and colleagues [50] recorded activity in a patient with intrathalamic depth electrodes as well as frontal cortex coverage with scalp EEG. They found that successful retrieval is associated with increased synchrony between the thalamus and PFC in the beta band as well as coupling between beta oscillations and gamma power. Indeed, it has been has proposed that coupling between the phase of lower frequencies and the amplitude of higher frequencies enhances local cortical processing, facilitating transmission of information across synchronized brain regions (Box 1) [51,52].
Intracranial recordings and reinstatement
Episodic retrieval often involves the reinstatement of neural activity patterns elicited during encoding [53]. This phenomenon is frequently studied using fMRI, limiting precise timing of these spatially localized patterns. Several ECoG studies report data from both the encoding and retrieval phases of long-term memory paradigms. Kucewicz et al. [38] recorded 50-500 Hz activity and reported a dissociation such that encoding of pictures induces more oscillatory activity in the occipital and parahippocampal cortices than retrieval, consistent with a model of bottom-up visual processing. Retrieval, in contrast, induces more high-frequency activity in the temporal and frontal cortices than encoding, consistent with top-down cognitive processing. Ekstrom and colleagues [54] recorded SUA and local field potentials in the hippocampus and entorhinal cortex during a navigation task and at subsequent recognition. Their data reveal a dissociation between SUA and power increases in broadband potentials, theta and gamma (30-100 Hz) bands, with increased SUA at encoding and increased local field potential activity at retrieval. Reinstatement may be specific to a subset of neurons in these MTL regions.
Context reinstatement – i.e., overlap of perceptual, conceptual, and/or categorical details based on similarity between encoding and retrieval – is evident in association cortices. Manning and others identified semantic components of neural activity during the encoding of words and reported that the resultant power spectra are reactivated in temporal and frontal cortices according to semantic clustering on subsequent free recall [55-57]. Overlap in neural activity between encoding and recall is not only similar, but also predictive of recall of items similar in context [55,56]; this pattern is more evident in the cortex than in the hippocampus [57]. Morton et al. [56] further reported that reactivation occurs at all frequencies studied (2-100 Hz) in the temporal cortex but does not occur at any frequency in the occipital cortex. Finally, these studies as well as [29] and [42] reported that reactivation of neural activity observed at encoding occurs just prior to retrieval, revealing that context reinstatement may be part of preparing to retrieve information and not part of retrieval itself.
Open questions and directions in intracranial recordings and memory
Intracranial recordings offer unparalleled spatiotemporal resolution in the study of human memory and capture high-frequency band responses, effectively bridging the study of memory across human and animal species, and raising the possibility of answering important, unresolved questions in the neuroscience of memory. ECoG studies of working and episodic memory in humans demonstrate the relationships between neuronal spiking and high-frequency activity with oscillations of different frequency bands, which regions interact to support memory and how these regions interact at different stages of memory processing, and the precise source of ERPs generated in memory performance. Recent ECoG data suggest that the PFC is a key hub for successful encoding in humans [24], providing evidence that the frontal cortex plays a domaingeneral, causal mechanism in memory networks, Furthermore, emerging subcortical depth recordings in both animals and humans suggest that the driving source of neocortical oscillations may be thalamic [50,58].
Subdural and depth recordings also shed light on multiple questions posed in the psychology of memory. For instance, ECoG provides support on the neural level for multiple models of recollection- versus familiarity-based retrieval [15-17], and Hanslmayr and Staudigl [59] argued that encoding and retrieval data support Endel Tulving’s principle of encoding specificity [60]. ECoG data also indicate that the relationship between successful encoding and retrieval operations – i.e., reinstatement – is both spatially and temporally complex.
Intracranial recordings will also provide guidance on how to create stimulation protocols to facilitate function in the damaged brain (see [27,61]). For instance, what is the precise nature of MTL-PFC interactions, and which mechanisms of inter-regional interaction play a causal role in successful memory formation and/or retrieval? Much of the extant neocortical data support a role of the PFC in the representation of encoded information, but suggest that the PFC may be a hub for multiplexing and successful encoding of stimuli (see [24]). Might the PFC support memory processes in a domain-general way, irrespective of the type of encoding modality or retrieval operation? Finally, because of its fine spatiotemporal resolution, ECoG offers the means for determining the oscillatory and phase parameters of potential therapeutic stimulation, as well as the precise location and timing of application to best facilitate function (also [61]). If there is a causal and domain-general frontal mechanism governing memory function, and if the source of that mechanism is indeed thalamic, these regions may present alternative stimulation sites – allowing possibility of memory facilitation for patients with MTL damage.
Highlights.
The spatiotemporal resolution of ECoG is unmatched in the study of human cognition.
Memory is linked to patterns of regional synchronization and desynchronization.
Frequency multiplexing occurs in hippocampal and cortical networks in memory.
High-frequency activity is central to successful memory encoding and retrieval.
Frontal cortex activity may play a central role in human memory networks.
Acknowledgments
We would like to thank Dr. Noa Ofen for valuable contributions in the planning of this piece. This research was supported by National Institute of Neurological Disorders and Stroke (NINDS) Grant 2R37NS21135 and the Nielsen Corporation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Elizabeth L. Johnson, Email: eljohnson@berkeley.edu.
Robert T. Knight, Email: rtknight@berkeley.edu.
References
-
••
Outstanding interest references
-
•
Special interest references
- 1.Dickerson BC, Eichenbaum H. The episodic memory system: Neurocircuitry and disorders. Neuropsychopharmacology. 2010;35(1):86–104. doi: 10.1038/npp.2009.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Scoville WB, Milner B. Loss of recent memory after bilateral hippocampal lesions. Journal of Neurology, Neurosurgery, and Psychiatry. 1957;20(11):11–21. doi: 10.1136/jnnp.20.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goldman-Rakic P. Cellular basis of working memory. Neuron. 1995;14:477–485. doi: 10.1016/0896-6273(95)90304-6. [DOI] [PubMed] [Google Scholar]
- 4.Müller NG, Knight RT. The functional neuroanatomy of working memory: Contributions of human brain lesion studies. Neuroscience. 2006;139:51–58. doi: 10.1016/j.neuroscience.2005.09.018. [DOI] [PubMed] [Google Scholar]
- •5.Burke JF, Long NM, Zaghloul KA, Sharan AD, Sperling MR, Kahana MJ. Human intracranial high-frequency activity maps episodic memory formation in space and time. NeuroImage. 2014;85:834–843. doi: 10.1016/j.neuroimage.2013.06.067. Using subdural and depth recordings in a cohort of 98 patients, the authors report that the neural regions identified in functional magnetic resonance imaging (fMRI) as associated with successful encoding fall into two spatiotemporally distinct neural networks – likely reflecting bottom-up perceptual processing and top-down control, respectively. This large-scale study demonstrates how intracranial recordings expand on traditional highspatial resolution imaging methods available to study human memory. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Paller KA, Wagner AD. Observing the transformation of experience into memory. Trends in Cognitive Sciences. 2002;6(2):93–102. doi: 10.1016/s1364-6613(00)01845-3. [DOI] [PubMed] [Google Scholar]
- 7.Jacobs J, Kahana MJ, Ekstrom AD, Fried I. Brain oscillations control timing of single-neuron activity in humans. Journal of Neuroscience. 2007;27(14):3839–3844. doi: 10.1523/JNEUROSCI.4636-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Axmacher N, Elger CE, Fell J. Working memory-related hippocampal deactivation interferes with long-term memory formation. Journal of Neuroscience. 2009;29(4):1052–1060. doi: 10.1523/JNEUROSCI.5277-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fernández G, Effern A, Grunwald T, Pezer N, Lehnertz K, Dümpelmann M, Van Roost D, Elger CE. Real-time tracking of memory formation in the human rhinal cortex and hippocampus. Science. 1999;285(5433):1582–1585. doi: 10.1126/science.285.5433.1582. [DOI] [PubMed] [Google Scholar]
- 10.Mormann F, Fernández G, Klaver P, Weber B, Elger CE, Fell J. Declarative memory formation in hippocampal sclerosis: An intracranial event-related potentials study. NeuroReport. 2007;18(4):317–321. doi: 10.1097/WNR.0b013e3280287ae9. [DOI] [PubMed] [Google Scholar]
- 11.Paller KA, McCarthy G. Field potentials in the human hippocampus during the encoding and recognition of visual stimuli. Hippocampus. 2002;12(3):415–420. doi: 10.1002/hipo.10053. [DOI] [PubMed] [Google Scholar]
- 12.Mormann F, Fell J, Axmacher N, Weber B, Lehnertz K, Elger CE, Fernández G. Phase/amplitude reset and theta-gamma interaction in the human medial temporal lobe during a continuous word recognition memory task. Hippocampus. 2005;15(7):890–900. doi: 10.1002/hipo.20117. [DOI] [PubMed] [Google Scholar]
- 13.Fell J, Ludowig E, Rosburg T, Axmacher N, Elger CE. Phase-locking within human mediotemporal lobe predicts memory formation. NeuroImage. 2008;43(2):410–419. doi: 10.1016/j.neuroimage.2008.07.021. [DOI] [PubMed] [Google Scholar]
- 14.Axmacher N, Cohen MX, Fell J, Haupt S, Dümpelmann M, Elger CE, Schlaepfer TE, Lenartz D, Sturm V, Ranganath C. Intracranial EEG correlates of expectancy and memory formation in the human hippocampus and nucleus accumbens. Neuron. 2010;65(4):541–549. doi: 10.1016/j.neuron.2010.02.006. [DOI] [PubMed] [Google Scholar]
- 15.Yonelinas AP. The nature of recollection and familiarity: A review of 30 years of research. Journal of Memory and Language. 2002;46(3):441–517. [Google Scholar]
- •16.Staresina BP, Fell J, Dunn JC, Axmacher N, Henson RN. Using statetrace analysis to dissociate the functions of the human hippocampus and perirhinal cortex in recognition memory. PNAS. 2013;110(8):3119–3124. doi: 10.1073/pnas.1215710110. The authors use medial temporal lobe depth recordings to reveal that the hippocampus and perirhinal cortex support qualitatively dissociable processes in successful item and source recognition, and that the hippocampus is sensitive to retrieval demands. These results show support on the neural level (with event-related potentials) for multiple models of memory retrieval and address a long-standing debate in the psychology and neuroscience of human memory. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rutishauser U, Schuman EM, Mamelak A. Activity of human hippocampal and amygdala neurons during retrieval of declarative memories. PNAS. 2008;105(1):329–334. doi: 10.1073/pnas.0706015105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Düzel E, Penny WD, Burgess N. Brain oscillations and memory. Current Opinion in Neurobiology. 2010;20(2):143–149. doi: 10.1016/j.conb.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 19.Rutishauser U, Ross IB, Mamelak AN, Schuman EM. Human memory strength is predicted by theta-frequency phase-locking of single neurons. Nature. 2010;464(7290):903–907. doi: 10.1038/nature08860. [DOI] [PubMed] [Google Scholar]
- 20.Matsumoto JY, Stead M, Kucewicz MT, Matsumoto AJ, Peters PA, Brinkmann BH, Danstrom JC, Goerss SJ, Marsh WR, Meyer FB, Worrell GA. Network oscillations modulate interictal epileptiform spike rate during human memory. Brain. 2013;136:2444–2456. doi: 10.1093/brain/awt159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rizzuto DS, Madsen JR, Bromfield EB, Schulze-Bonhage A, Seelig D, Aschenbrenner-Scheibe R, Kahana MJ. Reset of human neocortical oscillations during a working memory task. PNAS. 2003;100(13):7931–7936. doi: 10.1073/pnas.0732061100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Long NM, Burke JF, Kahana MJ. Subsequent memory effect in intracranial and scalp EEG. NeuroImage. 2014;84:488–494. doi: 10.1016/j.neuroimage.2013.08.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sederberg PB, Kahana MJ, Howard MW, Donner EJ, Madsen JR. Theta and gamma oscillations during encoding predict subsequent recall. Journal of Neuroscience. 2003;23(34):10809–10814. doi: 10.1523/JNEUROSCI.23-34-10809.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ••24.Burke JF, Zaghloul KA, Jacobs J, Williams RB, Sperling MR, Sharan AD, Kahana MJ. Synchronous and asynchronous theta and gamma activity during episodic memory formation. Journal of Neuroscience. 2013;33(1):292–304. doi: 10.1523/JNEUROSCI.2057-12.2013. Based on subdural and depth recordings in a cohort of 68 patients, the authors propose that successful encoding is linked to two major types of widespread-network spectral modulations: those reflecting local synchrony, and those that reflect either local synchrony concurrent with global asynchrony across regions or asynchronous modulations of neural activity. This study builds on previous electrophysiological work to show the important role of asynchronous activity in memory formation, and suggests a key role for the frontal cortex in human memory networks. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sederberg PB, Schulze-Bonhage A, Madsen JR, Bromfield EB, McCarthy DC, Brandt A, Tully MS, Kahana MJ. Hippocampal and neocortical gamma oscillations predict memory formation in humans. Cerebral Cortex. 2007b;17(5):1190–1196. doi: 10.1093/cercor/bhl030. [DOI] [PubMed] [Google Scholar]
- 26.Buzsáki G, Anastassiou CA, Koch C. The origin of extracellular fields and currents--EEG, ECoG, LFP and spikes. Nature Reviews Neuroscience. 2012;13(6):407–420. doi: 10.1038/nrn3241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Suthana N, Haneef Z, Stern J, Mukamel R, Behnke E, Knowlton B, Fried I. Memory enhancement and deep-brain stimulation of the entorhinal area. New England Journal of Medicine. 2012;366(20):502–510. doi: 10.1056/NEJMoa1107212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fell J, Ludowig E, Staresina BP, Wagner T, Kranz T, Elger CE, Axmacher N. Medial temporal theta/alpha power enhancement precedes successful memory encoding: Evidence based on intracranial EEG. Journal of Neuroscience. 2011;31(14):5392–5397. doi: 10.1523/JNEUROSCI.3668-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lega BC, Jacobs J, Kahana MJ. Human hippocampal theta oscillations and the formation of episodic memories. Hippocampus. 2012;22(4):748–761. doi: 10.1002/hipo.20937. [DOI] [PubMed] [Google Scholar]
- ••30.Watrous AJ, Tandon N, Conner CR, Pieters T, Ekstrom AD. Frequencyspecific network connectivity increases underlie accurate spatiotemporal memory retrieval. Nature Neuroscience. 2013;16(3):349–356. doi: 10.1038/nn.3315. Using subdural recordings across widespread medial temporal, frontal, and parietal regions, the authors show that retrieval of spatial and temporal context information is associated with network activity across these regions, hubbed in the medial temporal lobe. Importantly, spatial memory retrieval is linked to 1-4 Hz oscillations while temporal memory retrieval is linked to 7-10 Hz oscillations, showing that frequency multiplexing occurs in neural networks during successful memory retrieval. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Knight RT, Eichenbaum H. Multiplexed memories: A view from human cortex. Nature Neuroscience. 2013;16(3):257–258. doi: 10.1038/nn.3341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Anderson KL, Rajagovindan R, Ghacibeh GA, Meador KJ, Ding M. Theta oscillations mediate interaction between prefrontal cortex and medial temporal lobe in human memory. Cerebral Cortex. 2010;20(7):1604–1612. doi: 10.1093/cercor/bhp223. [DOI] [PubMed] [Google Scholar]
- 33.Foster BL, Kaveh A, Dastjerdi M, Miller KJ, Parvizi J. Human retrosplenial cortex displays transient theta phase locking with medial temporal cortex prior to activation during autobiographical memory retrieval. Journal of Neuroscience. 2013;33(25):10439–10446. doi: 10.1523/JNEUROSCI.0513-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Steinvorth S, Wang C, Ulbert I, Schomer D, Halgren E. Human entorhinal gamma and theta oscillations selective for remote autobiographical memory. Hippocampus. 2010;20(1):166–173. doi: 10.1002/hipo.20597. [DOI] [PubMed] [Google Scholar]
- 35.Ray S, Maunsell JHR. Different origins of gamma rhythm and high-gamma activity in macaque visual cortex. PLoS Biology. 2011;9(4):e1000610. doi: 10.1371/journal.pbio.1000610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cardin JA, Carlén M, Meletis K, Knoblich U, Zhang F, Deisseroth K, Tsai LH, Moore CI. Driving fast-spiking cells induces gamma rhythm and controls sensory responses. Nature. 2009;459:663–667. doi: 10.1038/nature08002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jacobs J, Kahana MJ. Neural representations of individual stimuli in humans revealed by gamma-band electrocorticographic activity. Journal of Neuroscience. 2009;29(33):10203–10214. doi: 10.1523/JNEUROSCI.2187-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ••38.Kucewicz MT, Cimbalnik J, Matsumoto JY, Brinkmann BH, Bower MR, Vasoli V, Sulc V, Meyer F, Marsh WR, Stead SM, Worrell GA. Highfrequency oscillations are associated with cognitive processing in human recognition memory. Brain. 2014 doi: 10.1093/brain/awu149. The authors test the role of oscillations in multiple high-frequency bands, ranging from 50-500 Hz, in subdural and depth recordings of successful encoding and retrieval. The data reveal that encoding pictures is linked to activity along the bottom-up visual processing stream – with distinct patterns detected in limbic and neocortical regions – and retrieval is linked to association cortices, likely reflecting cognitive control. This study demonstrates the role of fast activity and network synchronization in memory. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.van Vugt MK, Schulze-Bonhage A, Sekuler R, Litt B, Brandt A, Baltuch G, Kahana MJ. Intracranial electroencephalography reveals two distinct similarity effects during item recognition. Brain Research. 2009;1299:33–44. doi: 10.1016/j.brainres.2009.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- •40.Park J, Lee H, Kim T, Park G, Lee E, Baek S, Ku J, Kim IY, Kim SI, Jang DP, Kang JK. Role of low- and high-frequency oscillations in the human hippocampus for encoding environmental novelty during a spatial navigation task. Hippocampus. 2014 doi: 10.1002/hipo.22315. Based on hippocampal depth recordings taken during multiple sessions of virtual navigation, the authors show a double-dissociation between lower- and higher-frequency oscillations such that delta and theta activity increases incrementally as environments become less novel, while low-gamma activity decreases. High-gamma (51-100 Hz) activity decreases only in sessions of high recall success, revealing a specific role for high-gamma oscillations in successful encoding. [DOI] [PubMed] [Google Scholar]
- 41.Khursheed F, Tandon N, Tertel K, Pieters TA, Disano MA, Ellmore TM. Frequency-specific electrocorticographic correlates of working memory delay period fMRI activity. NeuroImage. 2011;56(3):1773–1782. doi: 10.1016/j.neuroimage.2011.02.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sederberg PB, Schulze-Bonhage A, Madsen JR, Bromfield EB, Litt B, Brandt A, Kahana MJ. Gamma oscillations distinguish true from false memories. Psychological Science. 2007;18(11):927–932. doi: 10.1111/j.1467-9280.2007.02003.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Foster BL, Dastjerdi M, Parvizi J. Neural populations in human posteromedial cortex display opposing responses during memory and numerical processing. PNAS. 2012;109(38):15514–15519. doi: 10.1073/pnas.1206580109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Axmacher N, Henseler MM, Jensen O, Weinreich I, Elger CE, Fell J. Cross-frequency coupling supports multi-item working memory in the human hippocampus. PNAS. 2010;107(7):3228–3233. doi: 10.1073/pnas.0911531107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Roux F, Uhlhaas PJ. Working memory and neural oscillations: Alpha-gamma versus theta-gamma codes for distinct WM information? Trends in Cognitive Sciences. 2014;18(1):16–25. doi: 10.1016/j.tics.2013.10.010. [DOI] [PubMed] [Google Scholar]
- 46.Lisman JE, Jensen O. The θ-γ neural code. Neuron. 2013;77(6):1002–1016. doi: 10.1016/j.neuron.2013.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fell J, Klaver P, Elfadil H, Schaller C, Elger CE, Fernandez G. Rhinalhippocampal theta coherence during declarative memory formation: Interaction with gamma synchronization? European Journal of Neuroscience. 2003;17(5):1082–1088. doi: 10.1046/j.1460-9568.2003.02522.x. [DOI] [PubMed] [Google Scholar]
- 48.Fell J, Klaver P, Lehnertz K, Grunwald T, Schaller C, Elger CE, Fernández G. Human memory formation is accompanied by rhinal–hippocampal coupling and decoupling. Nature Neuroscience. 2001;4:1259–1264. doi: 10.1038/nn759. [DOI] [PubMed] [Google Scholar]
- 49.Fell J, Axmacher N. The role of phase synchronization in memory processes. Nature Reviews Neuroscience. 2011;12(2):105–118. doi: 10.1038/nrn2979. [DOI] [PubMed] [Google Scholar]
- 50.Staudigl T, Zaehle T, Voges J, Hanslmayr S, Esslinger C, Hinrichs H, Schmitt FC, Heinze HJ, Richardson-Klavehn A. Memory signals from the thalamus: Early thalamocortical phase synchronization entrains gamma oscillations during long-term memory retrieval. Neuropsychologia. 2012;50(14):3519–3527. doi: 10.1016/j.neuropsychologia.2012.08.023. [DOI] [PubMed] [Google Scholar]
- 51.Canolty RT, Knight RT. The functional role of cross-frequency coupling. Trends in Cognitive Sciences. 2010;14(11):506–515. doi: 10.1016/j.tics.2010.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sadeh B, Szczepanski SM, Knight RT. Oscillations and behavior: The role of phase–amplitude coupling in cognition. In: Mangun G, editor. Cognitive Electrophysiology of Attention: Signals of the Mind. Elsevier; 2014. pp. 268–282. [Google Scholar]
- 53.Rissman J, Wagner AD. Distributed representations in memory: Insights from functional brain imaging. Annual Review of Psychology. 2012;63:101–128. doi: 10.1146/annurev-psych-120710-100344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ekstrom AD, Viskontas I, Kahana MJ, Jacobs J, Upchurch K, Bookheimer S, Fried I. Contrasting roles of neural firing rate and local field potentials in human memory. Hippocampus. 2007;17:606–617. doi: 10.1002/hipo.20300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Manning JR, Polyn SM, Baltuch GH, Litt B, Kahana MJ. Oscillatory patterns in temporal lobe reveal context reinstatement during memory search. PNAS. 2011;108(31):12893–12897. doi: 10.1073/pnas.1015174108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- •56.Morton NW, Kahana MJ, Rosenberg EA, Baltuch GH, Litt B, Sharan AD, Sperling MR, Polyn SM. Category-specific neural oscillations predict recall organization during memory search. Cerebral Cortex. 2013;23(10):2407–2422. doi: 10.1093/cercor/bhs229. The authors show that patterns of category-specific oscillatory activity identified via depth recordings and scalp electroencephalography (EEG) during the encoding of words predict whether words will be successfully recalled and, furthermore, the clustering of words at recall. This study provides high-resolution spatiotemporal data in support of retrieved-context models of memory, demonstrating how category information may be used effectively to organize search in memory. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Manning JR, Sperling MR, Sharan A, Rosenberg EA, Kahana MJ. Spontaneously reactivated patterns in frontal and temporal lobe predict semantic clustering during memory search. Journal of Neuroscience. 2012;32(26):8871–8878. doi: 10.1523/JNEUROSCI.5321-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Saalmann YB, Pinsk MA, Wang L, Li X, Kastner S. The pulvinar regulates information transmission between cortical areas based on attention demands. Science. 2012;337:753–756. doi: 10.1126/science.1223082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hanslmayr S, Staudigl T. How brain oscillations form memories - A processing based perspective on oscillatory subsequent memory effects. NeuroImage. 2014;85:648–655. doi: 10.1016/j.neuroimage.2013.05.121. [DOI] [PubMed] [Google Scholar]
- 60.Tulving E, Thomson D. Encoding specificity and retrieval processes in episodic memory. Psychological Review. 1973;80(5):352–373. [Google Scholar]
- 61.Lee H, Fell J, Axmacher N. Electrical engram: How deep brain stimulation affects memory. Trends in Cognitive Sciences. 2013;17(11):574–584. doi: 10.1016/j.tics.2013.09.002. [DOI] [PubMed] [Google Scholar]
- 62.Durschmid S, Zaehle T, Kopitzki K, Schmitt FC, Voges J, Henze H, Knight RT, Hinrichs H. Phase-amplitude cross-frequency coupling in the human nucleus accumbens tracks action monitoring during cognitive control. Frontiers in Human Neuroscience. 2013;7:634. doi: 10.3389/fnhum.2013.00635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Durschmid S, Quandt F, Kramer U, Hinrichs H, Schulz R, Pannek H, Chang EF, Knight RT. Oscillatory dynamics track motor learning in human cortex. PLoS One. 2014;9(2):e89576. doi: 10.1371/journal.pone.0089576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Szczepanski SM, Crone NE, Kuperman RA, Auguste KI, Parvizi J, Knight RT. Dynamic changes in phase-amplitude coupling facilitate spatial attention control in fronto-parietal cortex. PLoS Biology. 12(8):e1001936. doi: 10.1371/journal.pbio.1001936. [DOI] [PMC free article] [PubMed] [Google Scholar]


