Figure 3.
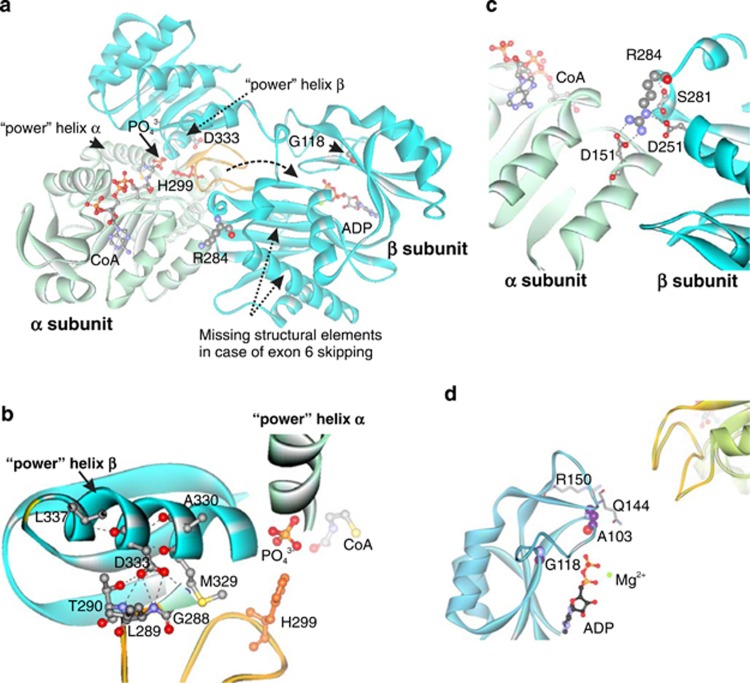
Modeled structure of human mitochondrial ADP-forming succinyl-CoA synthetase with labeled pathogenic mutations (a). α subunit: light green; β subunit: cyan; catalytic His299: red; phosphohistidine loop: orange. Dashed arrow: tentative movement of the phosphohistidine loop between active sites. Decomposition of succinyl-CoA onto succinate and CoA in the catalytic α-subunit requires participation of a phosphate ion, which is coordinated by the N-termini of the two ‘power' helixes: one from the α-subunit and the other from the β-subunit. This phosphate is utilized for phosphorylation of the catalytic His299 (His246 in E. coli) of the α-subunit located in the phosphohistidine loop.14 Phosphorylation of ADP takes place in the active site of the β-subunit. The movement of the phosphohistidine loop with phosphorylated catalytic histidine has been suggested to shuttle the phosphate between the active sites of both subunits.14, 15 The missing fragment in the case of skipping of exon 6 and part of exon 7, encompassing residues 221–267, contains Glu256, a critical residue for ADP phosphorylation.21 Location of Asp333, a part of ‘power' helix β and the interface between subunits, within the modeled human mitochondrial SCS-A (b). Hydrogen bonds formed by Asp333: black dashed lines. Location of Arg284 and Asp251 within the modeled human SCS-A (c). Arg284 localizes to the interface between two subunits and forms a number of contacts with neighboring residues (Ser281, Asp251 and Asp151). Non-covalent bonds formed by Arg284: black dashed lines. Location of Gly118 and Ala103, in the ADP-binding site, with respect to bound ADP and the phosphohistidine loop (yellow) of subunit α of SCS-A (d).