Abstract
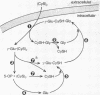
Cystine is a good acceptor of the gamma-glutamyl group of gamma-glutamyl donors in the reaction catalyzed by gamma-glutamyl transpeptidase. The product of the enzymatic reaction and an authentic sample of gamma-glutamylcystine were shown to exhibit identical chromatographic and electrophoretic behaviors; acid hydrolysis gave equimolar amounts of cystine and glutamate. In studies with two gamma-glutamyl donors, apparent Km values in the neighborhood of 0.3 mM were found for L-cystine; these values are not far from the concentrations of L-cystine in mammalian blood plasma. At an amino-acid acceptor concentration of about 0.5 mM, L-cystine is somewhat more active than L-glutamine, and much more active than L-cystein. L-gamma-Glutamyl-L-cystine was found to be a good substrate of gamma-glutamyl cyclotransferase. These observations thus indicate that L-cystine is a very active substrate of the gamma-glutamyl transpeptidase-gamma-glutamyl cyclotransferase pathway. In relation to the hypothesis that the gamma-glutamyl cycle functions in animo-acid transport, it may be significant that glutathione (which is the most abundant intracellular form) is a much better gamma-glutamyl donor than glutathione disulfide, while the predominant extracellular form-cystine-is a much better gamma-glutamyl acceptor substrate than cystein.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLACK S. THE BIOCHEMISTRY OF SULFUR-CONTAINING COMPOUNDS. Annu Rev Biochem. 1963;32:399–418. doi: 10.1146/annurev.bi.32.070163.002151. [DOI] [PubMed] [Google Scholar]
- Brigham M. P., Stein W. H., Moore S. THE CONCENTRATIONS OF CYSTEINE AND CYSTINE IN HUMAN BLOOD PLASMA. J Clin Invest. 1960 Nov;39(11):1633–1638. doi: 10.1172/JCI104186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COLMAN R. F., BLACK S. ON THE ROLE OF FLAVIN ADENINE DINUCLEOTIDE AND THIOL GROUPS IN THE CATALYTIC MECHANISM OF YEAST GLUTATHIONE REDUCTASE. J Biol Chem. 1965 Apr;240:1796–1803. [PubMed] [Google Scholar]
- CONN E. E., VENNESLAND B. Glutathione reductase of wheat germ. J Biol Chem. 1951 Sep;192(1):17–28. [PubMed] [Google Scholar]
- Crawhall J. C., Segal S. The intracellular ratio of cysteine and cystine in various tissues. Biochem J. 1967 Nov;105(2):891–896. doi: 10.1042/bj1050891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MAPSON L. W., GODDARD D. R. The reduction of glutathione by plant tissues. Biochem J. 1951 Oct;49(5):592–601. doi: 10.1042/bj0490592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meister A. Glutathione, metabolism and function via the gamma-glutamyl cycle. Life Sci. 1974 Jul 15;15(2):177–190. doi: 10.1016/0024-3205(74)90206-9. [DOI] [PubMed] [Google Scholar]
- Meister A. On the enzymology of amino acid transport. Science. 1973 Apr 6;180(4081):33–39. doi: 10.1126/science.180.4081.33. [DOI] [PubMed] [Google Scholar]
- Nickerson W. J., Romano A. H. Enzymatic Reduction of Cystine by Coenzyme I (DPNH). Science. 1952 Jun 20;115(2999):676–678. doi: 10.1126/science.115.2999.676. [DOI] [PubMed] [Google Scholar]
- Orlowski M., Meister A. The gamma-glutamyl cycle: a possible transport system for amino acids. Proc Natl Acad Sci U S A. 1970 Nov;67(3):1248–1255. doi: 10.1073/pnas.67.3.1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orlowski M., Richman P. G., Meister A. Isolation and properties of gamma-L-glutamylcyclotransferase from human brain. Biochemistry. 1969 Mar;8(3):1048–1055. doi: 10.1021/bi00831a036. [DOI] [PubMed] [Google Scholar]
- RACKER E. Glutathione reductase from bakers' yeast and beef liver. J Biol Chem. 1955 Dec;217(2):855–865. [PubMed] [Google Scholar]
- RACKER E. Glutathione-homocystine transhydrogenase. J Biol Chem. 1955 Dec;217(2):867–874. [PubMed] [Google Scholar]
- RALL T. W., LEHNINGER A. L. Glutathione reductase of animal tissues. J Biol Chem. 1952 Jan;194(1):119–130. [PubMed] [Google Scholar]
- ROMANO A. H., NICKERSON W. J. Cystine reductase of pea seeds and yeasts. J Biol Chem. 1954 May;208(1):409–416. [PubMed] [Google Scholar]
- SEMENZA G. Chromatographic purification of cysteinyl-glycinase. Biochim Biophys Acta. 1957 May;24(2):401–413. doi: 10.1016/0006-3002(57)90212-3. [DOI] [PubMed] [Google Scholar]
- Segal S., Crawhall J. C. Characteristics of cystine and cysteine transport in rat kidney cortex slices. Proc Natl Acad Sci U S A. 1968 Jan;59(1):231–237. doi: 10.1073/pnas.59.1.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal S., Smith I. Delineation of cystine and cysteine transport systems in rat kidney cortex by developmental patterns. Proc Natl Acad Sci U S A. 1969 Jul;63(3):926–933. doi: 10.1073/pnas.63.3.926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- States B., Segal S. Distribution of glutathione-cystine transhydrogenase activity in subcellular fractions of rat intestinal mucosa. Biochem J. 1969 Jun;113(2):443–444. doi: 10.1042/bj1130443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tate S. S., Meister A. Interaction of gamma-glutamyl transpeptidase with amino acids, dipeptides, and derivatives and analogs of glutathione. J Biol Chem. 1974 Dec 10;249(23):7593–7602. [PubMed] [Google Scholar]
- Tate S. S., Meister A. Stimulation of the hydrolytic activity and decrease of the transpeptidase activity of gamma-glutamyl transpeptidase by maleate; identity of a rat kidney maleate-stimulated glutaminase and gamma-glutamyl transpeptidase. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3329–3333. doi: 10.1073/pnas.71.9.3329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tietze F. Disulfide reduction in rat liver. I. Evidence for the presence of nonspecific nucleotide-dependent disulfide reductase and GSH-disulfide transhydrogenase activities in the high-speed supernatant fraction. Arch Biochem Biophys. 1970 May;138(1):177–188. doi: 10.1016/0003-9861(70)90297-3. [DOI] [PubMed] [Google Scholar]
- Tietze F. Disulfide reduction in rat liver. II. Chromatographic separation of nucleotide-dependent disulfide reductase and GSH-disulfide transhydrogenase activities of the high-speed supernatant fraction. Biochim Biophys Acta. 1970 Dec 16;220(3):449–462. doi: 10.1016/0005-2744(70)90276-7. [DOI] [PubMed] [Google Scholar]
- Van der Werf P., Orlowski M., Meister A. Enzymatic conversion of 5-oxo-L-proline (L-pyrrolidone carboxylate) to L-glutamate coupled with cleavage of adenosine triphosphate to adenosine diphosphate, a reaction in the -glutamyl cycle. Proc Natl Acad Sci U S A. 1971 Dec;68(12):2982–2985. doi: 10.1073/pnas.68.12.2982. [DOI] [PMC free article] [PubMed] [Google Scholar]