FIGURE 5.
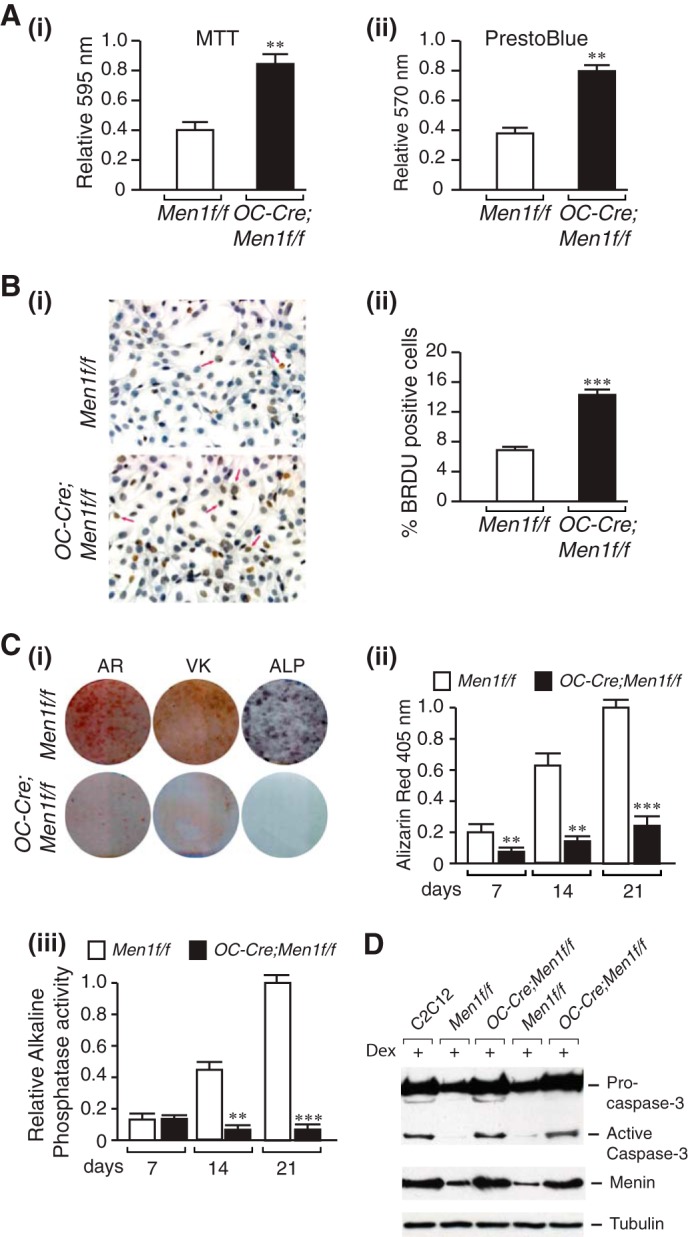
Increased proliferation, impaired mineral apposition, and ALP activity and increased susceptibility to apoptosis of menin-deficient calvarial osteoblasts. Primary osteoblasts were isolated from individual calvaria of 6-month-old Men1f/f (control) and OC-Cre;Men1f/f (osteoblast knock-out) mice and cultured for 7–10 days in αMEM, and the following assays were then carried out. A: i, MTT dye viability assay. Absorbance was read at 595 nm. Values shown are the mean ± S.E. (n = 3 independent experiments). **, p < 0.01. ii, PrestoBlue cell viability assay. Absorbance was read at 570 nm and normalized to the 600-nm value. Values shown are the mean ± S.E. (n = 3 independent experiments). **, p < 0.01. B, cell proliferation was assessed by BrdU incorporation. i, staining. ii, quantitation. ***, p < 0.001. C, Alizarin Red-S (AR) staining. Primary osteoblasts were plated in an osteogenic medium containing β-glycerophosphate and l-ascorbic acid. i, cells were fixed at 7, 14, and 21 days (shown) and stained with Alizarin Red-S. ii, quantitation was made by extraction and measurement of absorbance at 405 nm. Values are the mean ± S.E. (n = 3 independent experiments). **, p < 0.01. C: i, von Kossa (VK) staining. Primary osteoblasts were plated in an osteogenic medium. Phosphate deposition was evaluated at 14 and 21 days (shown) post-differentiation by staining fixed cells with von Kossa solution. Washed cells were scanned. C, ALP. Primary osteoblasts were cultured in an osteogenic medium for 7, 14, and 21 days (shown). i, ALP staining. Washed, fixed cells were overlaid with nitro blue tetrazolium chloride)/BCIP (5-bromo-4-chloro-3′-indolyl phosphate p-toluidine salt) (NBT) and incubated at room temperature for 20 min in the dark. iii, ALP activity. p-Nitrophenyl phosphate substrate was added to lysed cell extracts, and the reaction was stopped with 3 n NaOH. Absorbance was read at 405 nm, and values were normalized to protein concentration. Values are the mean ± S.E. (n = 3 independent experiments). **, p < 0.01; ***, p < 0.001. D, mouse C2C12 cells and adult primary osteoblasts originating from individual mice were seeded at a density of 1 × 106 in 60 mm plates. After 48 h cells were treated (+) or not with 1 mm dexamethasone (Dex) and incubated for a further 24 h. Proteins in cell lysates were separated by SDS-PAGE, transferred to PVDF membranes, and Western-blotted overnight at 4 °C with anti-caspase-3 antibody or anti-menin antibody followed by HRP-conjugated secondary antibody. Detection was performed using enhanced chemiluminescence. First lane, C2C12 cells; second and fourth lanes, Men1f/f osteoblasts; third and fifth lanes, OC-Cre;Men1f/f osteoblasts.
