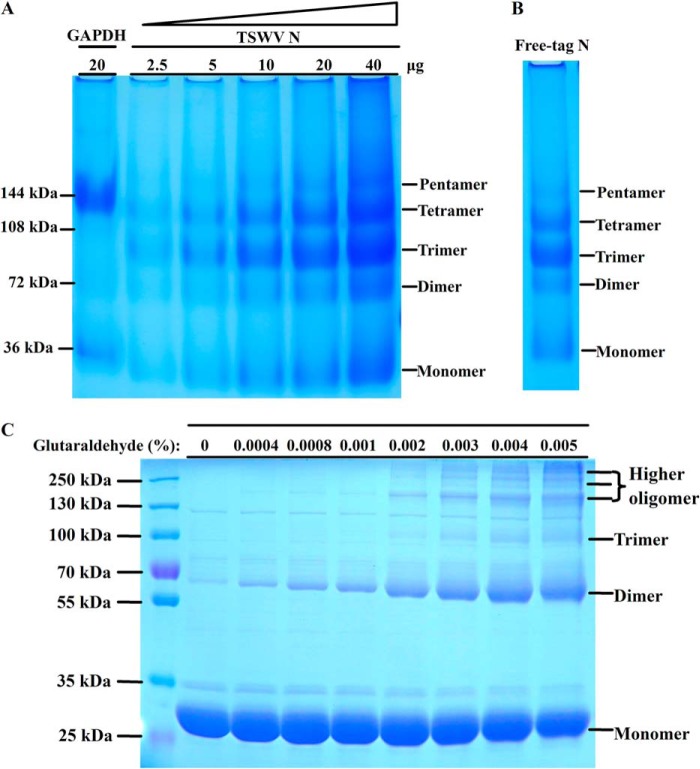
FIGURE 2.
Oligomerization state analysis of TSWV N protein. A, TSWV N protein forms a range of higher ordered oligomers in discontinuous blue native gel. Increasing amounts (2.5, 5, 10, 20, and 40 μg) of purified TSWV N were separated on a 4–16% discontinuous polyacrylamide gel. GAPDH (20 μg) was used as a size marker. B, discontinuous blue native PAGE analysis of oligomerization state of free-tag N. Approximately 20 μg of free-tag N was separated on a 4–16% discontinuous polyacrylamide gel. C, cross-linking of N protein by glutaraldehyde. The 30 μg of purified N protein was incubated with increasing amounts of glutaraldehyde. The mixtures were incubated at 37 °C for 5 min and then separated by 5–20% SDS-PAGE followed by Coomassie Blue staining. The protein marker was loaded on the same gel to mark the size of cross-linking products. Monomer, dimer, trimer, tetramer, and higher ordered oligomer positions are indicated in each gel.