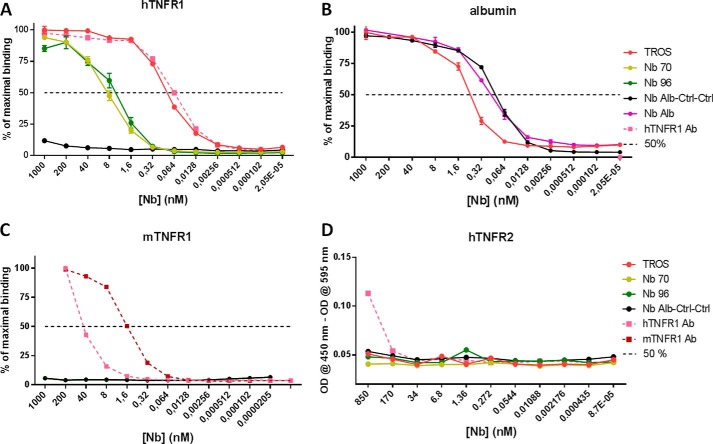
FIGURE 5.
A–D, binding affinity of nanobodies to hTNFR1 and albumin, cross-reactivity to mTNFR1, and hTNFR2 determined with ELISA. In all ELISAs, a 0.2 serial dilution of Nb was applied starting from 1 μm (and 850 nm in hTNFR2 ELISA). A, TROS and hTNFR1 Ab bind with equal affinities to hTNFR1, and affinities of Nb 96 and Nb 70 for hTNFR1 are lower. Generation of a multivalent Nb increased the affinity significantly. B, albumin binding was preserved in TROS and Nb Alb-Ctrl-Ctrl in comparison with Nb Alb. C–D, no cross-reactivity to mTNFR1 (C) or hTNFR2 (D) was observed for TROS, whereas hTNFR1 Ab did to mTNFR1 and hTNFR2. Nb Alb-Ctrl-Ctrl, an irrelevant control Nanobody; hTNFR1 Ab, a human TNFR1 antibody, positive control. 50%, Nanobody concentration that binds 50% of hTNFR1 or albumin. All ELISAs were done in triplicate, and data are represented as mean ± S.E.