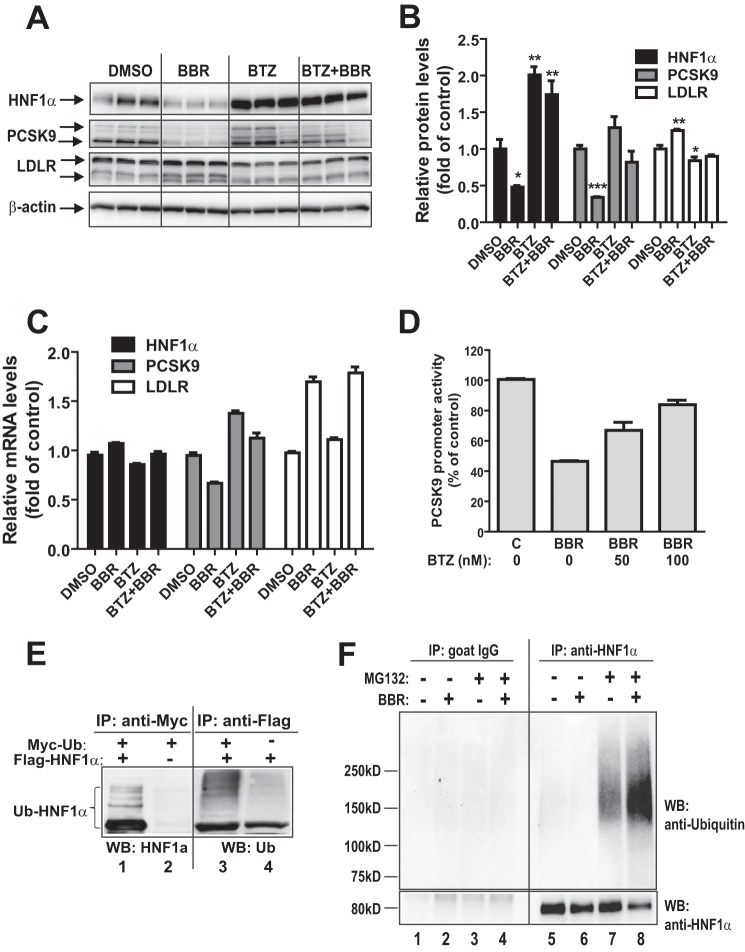
FIGURE 5.
Proteasomal pathway participates in BBR-induced degradation of HNF1α protein. A, HepG2 cells were treated with 40 μm BBR in the absence or the presence of BTZ (1 μm) for 16 h, and cell lysates were utilized for immunoblotting using anti-HNF1α, anti-PCSK9, and anti-LDLR antibodies. Triplicate wells were used for each treatment. The data shown are representative of three separate experiments with similar results. B, the protein abundances of HNF1α, PCSK9, and LDLR in A were quantified with the Alpha View software with normalization by signals of β-actin. The values are presented as means ± S.E. (error bars). *, p < 0.05; **, p < 0.01; ***, p < 0.001 compared with DMSO. C, HepG2 cells were treated with 40 μm BBR in the absence or the presence of BTZ for 16 h, and total RNA was isolated for qPCR analysis of mRNA levels of HNF1α, PCSK9, and LDLR. Duplicate wells were used for each treatment, and individual cDNA samples were measured in triplicates. The values are presented as mean ± S.E. The data shown are representative of two separate experiments with similar results. D, the PCSK9 promoter reporter construct pGL3-PCSK9-D4 was transiently cotransfected with pSV-β-gal vector into HepG2 cells. One day post transfection, cells were incubated in 0.5% FBS medium overnight, followed by a 24-h treatment of 40 μm BBR in the absence or the presence of BTZ at the indicated concentrations. Cells were harvested, and the luciferase and β-galactosidase activities were measured as described under “Experimental Procedures.” Normalized luciferase activity of untreated cells is expressed as 100%. The data shown are representative of three separate experiments with similar results. E, plasmids encoding FLAG-tagged HNF1α and Myc-tagged ubiquitin were cotransfected into HEK293 cells. The empty vectors of pCMV-entry and pCMV-Myc were transfected as a mock control. Cell lysates were harvested after 2 days of transfection and immunoprecipitated with anti-Myc or anti-FLAG antibodies. Ubiquitinated HNF1α was detected by immunoblotting (WB) using anti-Myc or anti-FLAG antibodies, respectively. The data shown are representative of three separate experiments with similar results. F, HepG2 cells were treated for 24 h with BBR or DMSO as a control. During the last 8 h of BBR treatment, MG132 (20 μm) or DMSO was added to cells. Total cell lysates were prepared, and 500 μg of proteins from total lysates were subjected to IP with anti-HNF1α antibody or control antibody goat IgG. IP complexes were analyzed for ubiquitinated HNF1α with anti-HNF1α antibody and anti-ubiquitin antibody. The data shown are representative of two separate experiments with similar results.