Figure 2.
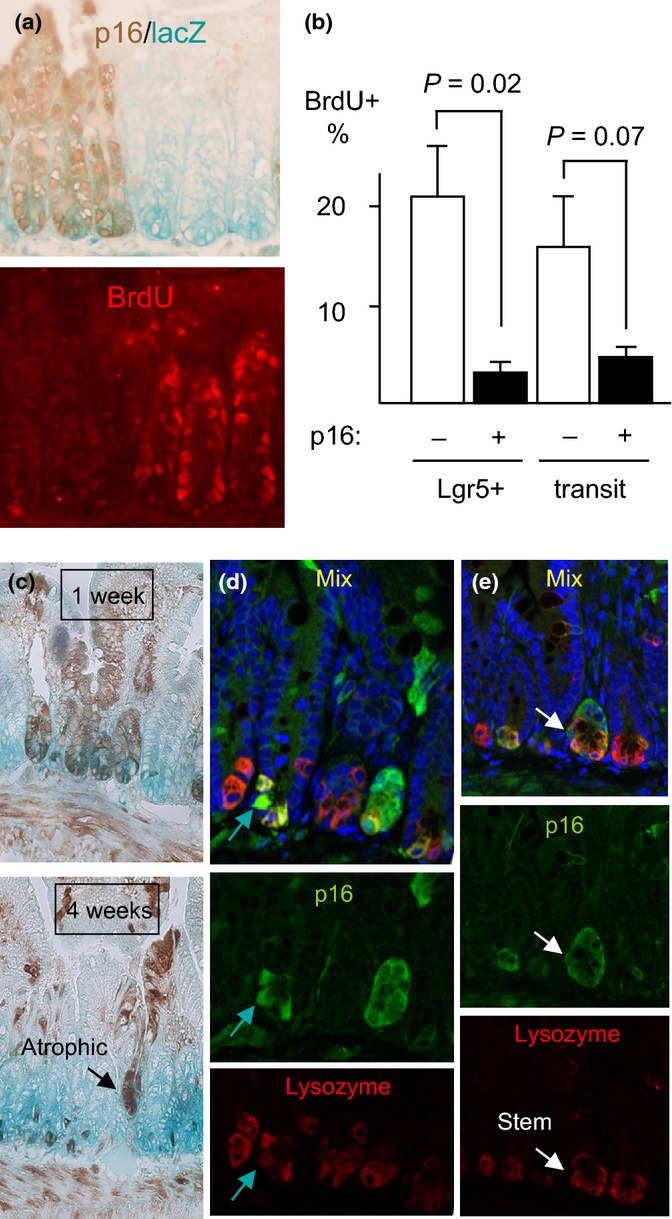
Induced p16 inhibits proliferation of intestinal stem cells. (a) CMV-rtTA:TetO-p16:Lgr5-lacZ mice were treated with Dox for 1 week and injected with BrdU. LacZ was detected by activity stain (left, aqua), p16 by IHC (left, brown), and BrdU in the same section by IF (right, red). Note paucity of BrdU staining in the p16+ Lgr5-lacZ+ cells (left 3 crypts). Across 8 20× fields, p16+ Lgr5+ cells (N = 331) showed no BrdU staining vs. 15% of the p16- Lgr5+ cells (N = 461, P = 0.02) (b) In the same mice, p16 and BrdU were detected by IHC in serial 4- to 5-micrometer sections and the % BrdU+ cells were scored in Lgr5+ and transit-amplifying cells, respectively. P values by two-sided t-tests. The one-sided t-test for transit cells = 0.03. N = 3 mice; 5803 cells counted. (c) Reduced p16 expression in small intestines of mice with continuous p16 induction. CMV-rtTA:TetO-p16:Lgr5-lacZ mice were treated with Dox near 2 mos of age for 1 or 4 weeks. IHC for p16 (brown) and activity assay for lacZ (aqua). Fields 10x. N = 3 mice per time point. Note the gradual loss of p16 expression, with preferential retention at the crypt base (P = 0.02 vs. transit-amplifying zones, P = 0.007 vs. villi). An atrophic, p16+ crypt is marked. (d, e) Partial costaining for p16 and lysozyme in crypt base cells, detected by confocal co-IF. Mice treated with Dox d20-40 were stained for p16 (green), lysozyme (red), and DNA (DAPI, blue; top). Green arrows (d) mark a p16+ cell that stains weakly for lysozyme, reflecting partial paneth cell differentiation. White arrows (e) identify a p16+ lysozyme- cell that is surrounded by lysozyme+ cells and, hence, is likely a stem cell.
