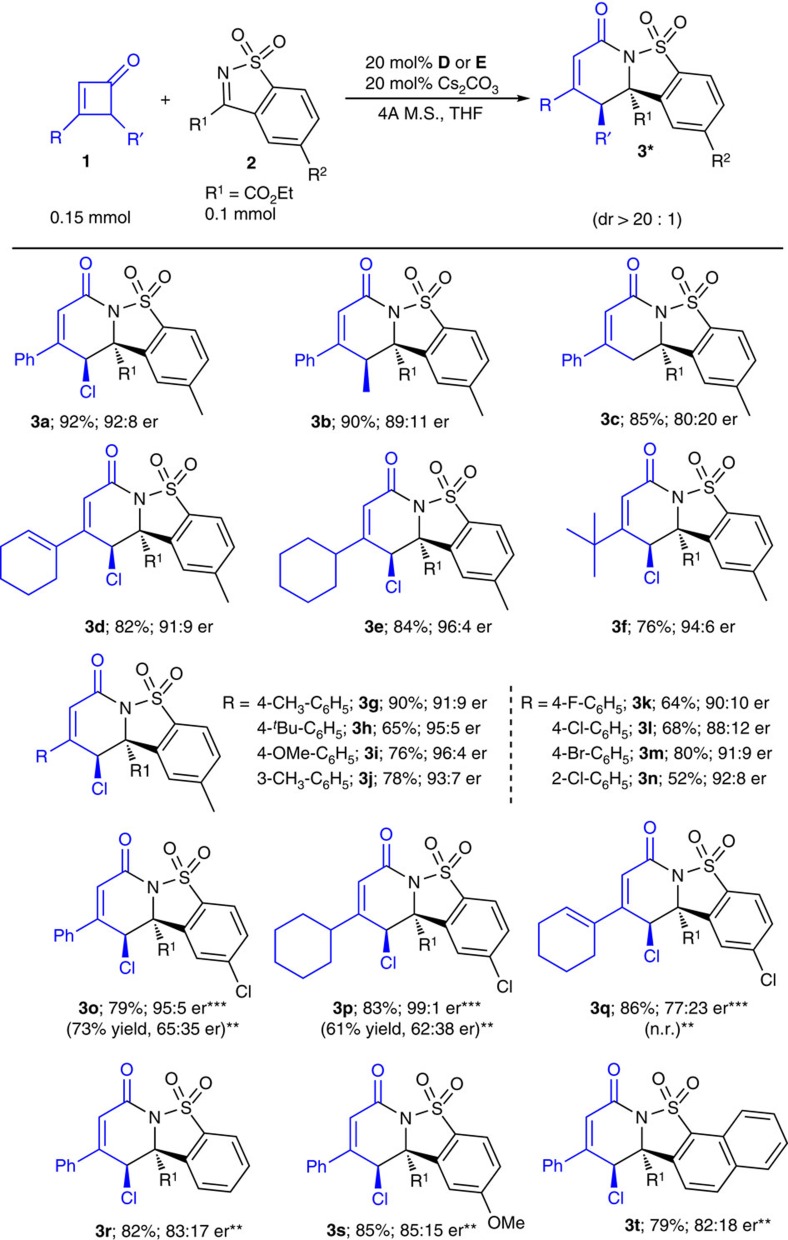
Figure 2. Reaction scope.
*The scope of this catalytic transformation was evaluated under standard conditions (Table 1, entry 7). Substrate scope includes γ- (3a–3c) and β-substituents (3d–3n) cyclobutenones (using 2a as the optimal imine), and various imines (3o–3t, using 1a as the optimal substrate). Reported yields were isolated yields of 3 based on imine 2. Diastereoselective ratio (dr of 3 was determined via 1H NMR analysis of the unpurified reaction mixture. Relative configuration of the major diastereoisomer was assigned based on X-ray structure of 3b and 3m (CCDC 988901, CCDC 988902, see Supplementary Information for more details). **The reactions were performed at 25 °C for 36 h. ***The reactions were performed using pre-catalyst E at 25 °C for 36 h (the reaction temperature was 0 °C for 3p).