Figure 1.
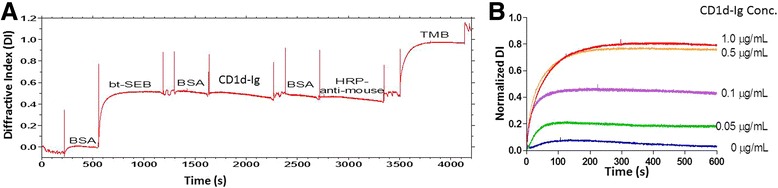
The kinetics and specificity of the capture of CD1d with biotinylated SEB (bt-SEB) with the dotReady system were characterized. A. Kinetics of SEB-CD1d interaction: A representative real-time trace depicts the kinetics of capturing CD1d with biotinylated SEB (bt-SEB) on the dotReady™ system. Here, the X-axis indicates the assay duration in seconds (s) and the Y-axis indicates measured diffractive index (DI). 10 μg/ml bt-SEB was immobilized on the avidin sensor during a 10 m incubation. CD1d:Ig fusion protein (extracellular major histocompatibility complex (MHC) class I-like domains of human CD1d fused with VH regions of mouse IgG1) (1 μg/ml) was added and incubated for a 10 m post-BSA treatment. After a second round of BSA washing, the complex was incubated with horse anti-mouse HRP-conjugated secondary antibody (HRP-anti-mouse; 1 μg/ml) for another 10 m. Finally, TMB was added as the reporting agent. The shift of DI with time in seconds is presented herein. For all DI tracing, the upward spikes are air gaps separating reagents. All non-labeled portions are attributed to the wash steps. Increased DI signals showing as an upward ramp indicated the binding of a reagent in this step. The highest ramp after TMB presentation suggests the successful binding of SEB and CD1d. B. Signal increases with increasing concentrations of CD1d presented to bind SEB: The assay followed the same sequential addition described in Figure 1A, namely, the presentation of biotinylated SEB to avidin sensor, followed by addition of CD1d:Ig fusion protein, horse anti-mouse HRP-conjugated secondary protein and TMB, respectively intercepted with BSA washing. The normalized DI signals measured during 600 s after the introduction of TMB were plotted against the serially increasing concentrations of CD1d from 0 to 1.0 μg/ml. A sigmoidal dose–response (variable slope) function was used to fit the curve, R2 = 0.9984. The positive correlation of the increased concentration of CD1d:Ig with enhanced DI while keeping the concentration of the other reagents constant validated the assay specificity.
