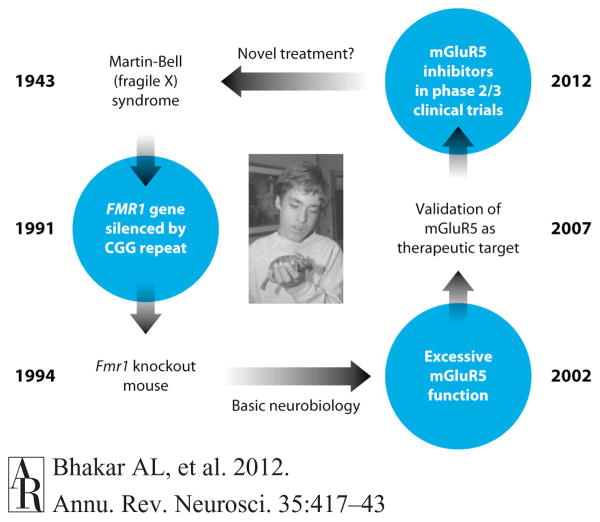
Figure 1.
Fulfilling the promise of molecular medicine in FX. Martin & Bell (1943) described a group of patients characterized by a common set of features that included intellectual disability and social withdrawal. The causative gene mutation was discovered in 1991 (Pieretti et al. 1991, Verkerk et al. 1991). The FMR1 gene on the X chromosome is silenced, and the protein FMRP is not produced. Shortly thereafter, the Fmr1-KO mouse model was generated (Dutch-Belgian Fragile X Consort. 1994) and has been intensively studied by neurobiologists interested both in the disease and FMRP. In 2002, it was discovered that a form of synaptic plasticity—mGluR LTD—was exaggerated in the Fmr1 KO mouse (Huber et al. 2002). This led to the mGluR theory of fragile X (Bear et al. 2004), which posits that many symptoms of the disease are due to exaggerated responses to activation of mGluR5. The theory was definitively validated in 2007 with the demonstration that multiple FX phenotypes are corrected in the Fmr1-KO mouse by genetic reduction of mGluR5 protein production (Dolen et al. 2007). In addition, numerous animal studies showed that pharmacological inhibition of mGluR5 ameliorates FX mutant phenotypes. In 2009, inhibitors of mGluR5 entered into human phase 2 trials (http://clinicaltrials.gov). If successful, these trials will represent the first pharmacological treatment for a neurobehavioral disorder that was developed from the bottom up: from gene discovery to pathophysiology in animals to novel therapeutics in humans. Abbreviations: CGG, cytosine-guanine-guanine; FMRP, fragile X mental retardation protein; FX, fragile X; mGluR5, metabotropic glutamate receptor 5; KO, knockout; LTD, long-term synaptic depression. Image courtesy of FRAXA Research Foundation, with permission.