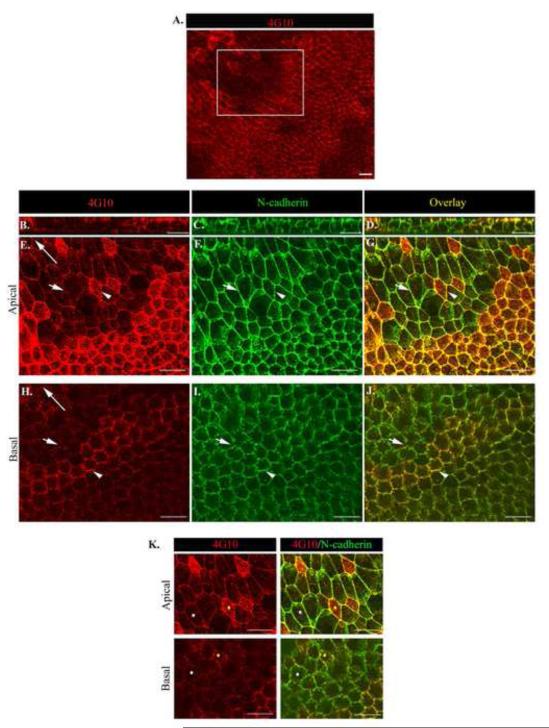
Figure 5. Tyrosine phosphorylation at junctional interfaces provide a mechanism to spatially regulate distinct apical/basal junctional remodeling during collective migration.
Wounded lens cultures were imaged by confocal microscopy at one day after injury following co-immunostaining for (A,B,E,H,K left panel) phosphotryosine (pY, 4G10) and (C,F I) N-cadherin and (D,G,J,K right panel) pY/N-cadherin overlay. Z-stacks were collected and shown as (B-D) orthogonal cuts or (A, E-K) single optical planes at the cells’ apical and basal domains. Low magnification image of the wounded epithelium (A) boxed region indicates the area depicted in (E-K). The cell-cell junctions of groups of neighboring cells were independently targeted by pY (E-K). White arrow head indicates an N-cad junction with increased pY staining vs. short white arrow depicting N-cadherin junction with less pY staining. To determine if cadherin junctions at the apical and basal domains were being targeted by pY at the same time, cells were marked by different color dots and followed in the apical to basal planes of the epithelium. Results reveal simultaneous increased pY targeting of apical and basal N-cad junctions (K, yellow dot). White dot follows N-cad junctions with less pY staining. Long arrows indicate the direction of migration. Mag. Bar = 20 μm. Studies are representative of at least three independent experiments.