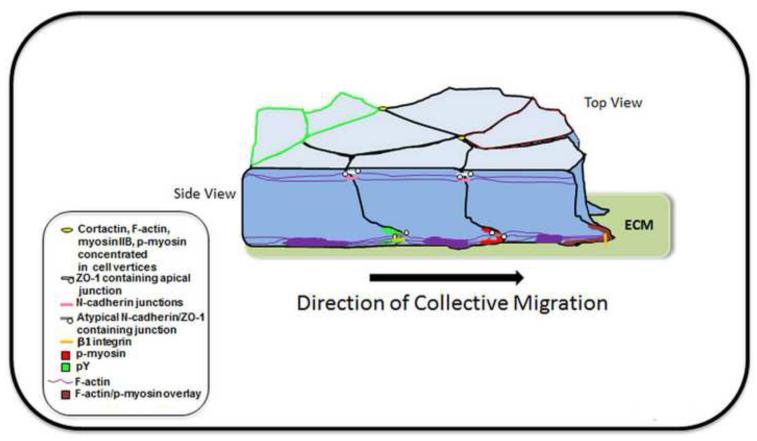
Figure 8. Model of the functional features of a wounded epithelium collectively moving in response to injury.
This model shows how cell behavior and function can be independently regulated at cells apical and basal domains through distinct junctional complexes and cytoskeletal organization to coordinate the collectivity of movement of an epithelium in response to injury. An apical N-cadherin/ZO-1 junctional complex linked at specific domains to pMyosin activity provides the mechanical forces that maintain integrity of the epithelial monolayer as it moves to repair the adjacent wounded area. The myosin IIB isoform has a role in this process at cell vertices, regions of contact between three or more cells, which together with pMyosin has the potential to create tension forces that provide stable reference points for cells to remodel and undergo rearrangements within the moving monolayer. The atypical N-cadherin/ZO-1 junctions we have discovered that are unique to the cryptic lamellipodia and the action of pMyosin with these crucial junctional structures are poised to drive the protrusive forces required for the forward, collective movement of a wounded epithelium. This mechanism is reflected in the coordinated pointing of all the cryptic lamellipodia in the direction of migration, and the basal domains of these cells preceding their apical domains into the wounded area. While β1 integrin is also localized to the cells’ basal surfaces of these migrating cells, its presence is much less prominent than the atypical N-cadherin/ZO-1 junctions, suggesting the importance of N-cadherin/ZO-1/pMyosin-linked protrusive forces for driving the forward, collective movement of an epithelium. Tyrosine phosphorylation, found discretely localized to both apical and basal junctional regions, provides a mechanism by which to spatially regulate junctional remodeling during injury repair. This model shows how distinct structure and myosin-linked function of cell-cell junctions at the apical and basal domains of injured epithelia coordinate effective wound repair.