Abstract
Rationale
Information on how ambient air pollution affects susceptible populations is needed to ensure protective air quality standards.
Objectives
To estimate the effect of community-level ambient particulate matter (PM) and ozone (O3) on respiratory symptoms among primarily African-American and Latino, lower-income asthmatic children living in Detroit, Michigan and to evaluate factors associated with heterogeneity in observed health effects.
Methods
A cohort of 298 children with asthma was studied prospectively from 1999 to 2002. For 14 days each season over 11 seasons, children completed a respiratory symptom diary. Simultaneously, ambient pollutant concentrations were measured at two community-level monitoring sites. Logistic regression models using generalized estimating equations were fit for each respiratory symptom in single pollutant models, looking for interactions by area or by corticosteroid use, a marker of more severe asthma. Exposures of interest were: daily concentrations of PM<10 μm, <2.5 μm, and between 10 and 2.5 μm in aerodynamic diameter (PM10, PM2.5, and PM10–2.5, respectively), the daily 8-hour maximum concentration of O3 (8HrPeak), and the daily 1-hour maximum concentration of O3 (1HrPeak).
Results
Outdoor PM2.5, PM10, 8HrPeak, and 1HrPeak O3 concentrations were associated with increased odds of respiratory symptoms, particularly among children using corticosteroid medication and among children living in the southwest community of Detroit. Similar patterns of associations were not seen with PM10–2.5.
Conclusions
PM2.5 and O3 at levels near or below annual standard levels are associated with negative health impact in this population of asthmatic children. Variation in effects within the city of Detroit and among the subgroup using steroids emphasizes the importance of spatially refined exposure assessment and the need for further studies to elucidate mechanisms and effective risk reduction interventions.
Keywords: Asthma, Child, Community-based participatory research, Particulate matter, Ozone, Vulnerable populations
1. Introduction
Ambient air pollution remains an important public health issue. Several gaseous and particulate pollutants at levels typically found in U.S. urban areas have been associated with a wide variety of negative health effects including exacerbation of pre-existing respiratory conditions (Gent et al., 2003; Mann et al., 2010; Mortimer et al., 2000), increased risk for cardiovascular disease and events (Dennekamp et al., 2010; Dominici et al., 2006; Sun et al., 2010), increased cardiopulmonary mortality (Brook et al., 2010), and increased all-cause mortality (Dominici et al., 2005). Air pollution may play a role in retarding normal lung growth among children (Gauderman et al., 2004; He et al., 2010) and is a potential cause of incident asthma (Gauderman et al., 2005).
Although, on average, air quality has improved in the U.S., the total number of people living in areas with at least one regulated pollutant exceeding standards, i.e., non-attainment areas, remains very high (United States Environmental Protection Agency [U.S. EPA], 2005a, 2005b). For example, in 2008, 59% of children in the U.S. lived in counties in which 8-hour air quality standards for O3 of 75 ppb were exceeded (U.S. EPA, 2010). Currently, attainment with air quality standards is assigned at the area or multi-county level. Increasingly, there is recognition that there may be important variation in pollution and differential health effects within urban geographies (Dvonch et al., 2009; Jerrett et al., 2005), suggesting that more refined spatial or temporal monitoring may be informative for understanding health risks.
Children with asthma are particularly vulnerable to air pollution exposure. Several studies have implicated ambient O3 and PM as triggers of asthma symptoms or acute changes in lung function (Dales et al., 2009; Ostro et al., 2001; Weinmayr et al., 2010). These populations may experience negative health impacts even when standards are met, and certain pollutants may be more responsible for these health effects. In addition, social and race/ethnic disparities in asthma morbidity as well as air pollution exposure have heightened interest in protecting the health of children living in relative socioeconomic disadvantage and in areas with substantial environmental exposures (Gold and Wright, 2005).
We assessed levels of ambient air pollutants and the role they play in exacerbating childhood asthma in Detroit, Michigan. In this project, 298 children with asthma were studied prospectively from 1999 to 2002. At the time of the study, the southeast region of Michigan was in non-attainment with the 8-hour O3 and annual PM2.5 standard, but was in compliance with the PM10 standard (U.S. EPA, 2005a, 2005b). In a previous analysis utilizing a subset of six seasons of data, we found that, in our population, children using maintenance corticosteroid therapy were most susceptible to acute worsening of lung function in association with fluctuations in levels of ambient PM10, PM2.5, andO3 (Lewis et al., 2005). The objectives of the current analysis were to assess whether exposure to community-level airborne particulate matter and ozone were also associated with respiratory symptoms and to explore whether subpopulations of children with asthma showed differential vulnerability to the effects of air pollution.
2. Methods
2.1. Community-based participatory research
This study was conducted by Community Action Against Asthma (CAAA), a community-based participatory research (CBPR) partnership that developed as an outgrowth of the Detroit Community-Academic Urban Research Center. Please see acknowledgements for the list of CAAA member organizations. Details of the CBPR principles and processes used in this study and discussion of the benefits and challenges of this approach are available elsewhere (Edgren et al., 2005; Israel et al., 2005, 2008; Parker et al., 2003).
2.2. Study population
Children with known or probable asthma living in two communities in Detroit (East and Southwest) with predominantly African-American and Hispanic populations were recruited using a screening questionnaire distributed through schools to identify children with symptoms consistent with active asthma (Lewis et al., 2004). Two hundred and ninety-eight children meeting inclusion criteria of age between 5 and 12 years, self-report of symptoms consistent with active asthma symptoms, and residence in either East or Southwest Detroit, were enrolled from the Fall 1999–Spring 2000 and followed prospectively until Spring 2002. Eighty-two percent reported a physician diagnosis of asthma, though this was not independently confirmed.
Enrolled children participated in two studies that were conducted simultaneously: a prospective epidemiologic study of the health effects of ambient air pollutants (Hammond et al., 2008; Keeler et al., 2002; Lewis et al., 2005; Yip et al., 2004), the subject of this report, and a randomized controlled trial of an in-home intervention to reduce exposure to indoor allergens, described elsewhere (Parker et al., 2008). Children were randomized to receive intensive home visits by community outreach workers, asthma environmental education, and environmental supplies during either the first or second year of study enrollment. Two hundred and thirty children (77%) were still enrolled after one year and 199 children were still participating at the end of the study period, for an overall 67% retention rate after nearly 3 years.
2.3. Data collection
2.3.1. Health outcomes
For 11 seasons from fall 1999 to spring 2002, for 14 consecutive days each season, children completed a daily ‘checkbox’ symptomdiary noting the presence or absence of specific respiratory symptoms (cough, wheeze, shortness of breath [SOB], chest tightness or heaviness, or waking up at night with respiratory symptoms). Covariate information was obtained from an annual in-person interview of the child’s primary caregiver. The analyses presented here utilized self-reported demographic information, asthma characteristics, medication use, and presence of environmental tobacco smoke as assessed at the baseline interview.
2.3.2. Air pollution
During these symptom reporting periods, concentrations of two airborne particle size fractions, PM10 and PM2.5, O3, and meteorologic variables were measured at two community-level monitoring sites established on the rooftops of representative schools in the east and southwest Detroit study areas. Twenty-four hour samples of PM were collected using Teflon-coated aluminum cyclone inlets and filter-pack assemblies (University Research Glassware, Carrboro, NC) with 2-micronpore 47 mm Teflon membrane filters (Pall Life Sciences, Ann Arbor, MI) at a flow rate of 16.7 L/min, and were analyzed in a class 100 clean laboratory (University of Michigan Air Quality Laboratory, Ann Arbor, MI). The limit of detection under these conditions was 0.2 μg/m3. PM10–2.5 was calculated as the difference of PM10 minus PM2.5. Ozone was measured during 8 seasons, recorded as 30-minute average values. These readings were used to calculate the maximum 8-hour and 1-hour average O3 concentrations. Ozone was not measured in the first two seasons (Fall 1999 and Winter 2000), or the subsequent winter (Winter 2001). Details of these exposure assessment methods have been previously described (Keeler et al., 2002). Five air pollution exposures were examined: Daily 24-hour concentrations (μg/m3) of PM2.5, PM10, PM10–2.5, maximum 8-hour, and maximum 1-hour average concentrations (ppb) of O3.
Over 95% of children lived within a 5-km radius of one of the monitors and, for the statistical analysis, was linked to the pollution measurements obtained from the monitor in their community. There was no geographic overlap between population or study areas.
2.4. Statistical methods
Data was initially examined using descriptive summary statistics and bivariate Pearson’s correlations. Multivariate associations between air pollution and health outcomes were assessed with logistic regression using generalized estimating equations (GEE) in combination with the alternating logistic regression (ALR) algorithm. GEE adjusts for correlation between repeated measures for an individual in a longitudinal design. The ALR algorithm models the association between pairs of binary responses with log odds ratios, instead of with correlations, proving more robust estimates of the standard error compared to standard GEE models. Thus, in our models of binary outcomes, the GEE with ALR models the log odds ratios.
Results are expressed as the odds ratio (OR) for every one interquartile range (IQR) increase in the overall exposure. Models investigated lagged exposures: exposure measured 1 day prior to the health outcome (Lag1), 2 days prior (Lag2), an average exposure 3–5 days before the outcome (Lag3–5), and an average exposure 1–5 days before the outcome (5DaysAve). We adjusted for the following potential confounding factors in all models: age, sex, location (Eastside or Southwest), race, household income, smoker in the home, season (modeled as 3 indicator variables, with winter as the reference), and variables indicating group status in the companion home intervention study (control or intervention), time (pre- or post-intervention), and the interaction between intervention group status and time. We considered models that assessed effect modification by maintenance corticosteroid use, and separately, by community location. (See Supplemental information for additional details.)
3. Results
3.1. Population
The 298 children were, on average, almost 9 years old at enrollment, and were predominantly male (Table 1). The majority self-identified as either African-American or Latino/Hispanic. More children participating in our study lived on the Eastside of Detroit than the Southwest, reflecting the relative geographic dimensions of these areas. Most children came from families with annual household incomes less than $20,000. About half of the children reported having symptoms consistent with moderate to severe persistent asthma. Despite the high proportion of children in our study with persistent asthma of any severity, only 9.7% reported maintenance corticosteroid use.
Table 1.
Characteristics of children reported on baseline caregiver interview.
| Characteristic | Subgroup | Full samplea | Eastside | Southwest | p-value |
|---|---|---|---|---|---|
|
|
|
|
|||
| N=298 | N=223 | N=75 | |||
| Child’s age in years at Sept. 30, 1999, mean (sd) | 8.92 (1.46) | 9.02 (1.46) | 8.06 (1.42) | 0.03 | |
| Gender, % | Female | 42 | 43 | 40 | 0.69 |
| Child’s ethnicity, % | African-American/Black | 80.87 | 95.5 | 37.3 | <0.001 |
| Hispanic/Latino/Spanish | 10.4 | 0 | 41.33 | ||
| Other | 8.7 | 4.5 | 21.37 | ||
| Child’s location of residence, % | Eastside | 74.83 | |||
| Southwest | 25.17 | ||||
| Caregiver education, % | 1–11 grade | 37.3 | 35 | 44 | 0.13 |
| High school graduate/GED | 33.9 | 33.2 | 36 | ||
| Any college | 28.77 | 31.8 | 20 | ||
| Household annual income, % | Less than $10,000 | 45.5 | 44.3 | 49.2 | 0.71 |
| $10,001–$20,000 | 31.6 | 32.9 | 27.7 | ||
| $20,001 or more | 22.9 | 22.9 | 23.1 | ||
| Caregiver smokes cigarettes (self-report), % | Smoke | 38 | 37.8 | 38.7 | 0.88 |
| Any household member smokes cigarettes, % | One or more smokers | 55.41 | 56.6 | 52 | 0.49 |
| Asthma severity, % | Moderate to severe | 47.65 | 45.7 | 53.3 | 0.47 |
| Mild persistent | 28.19 | 29.6 | 24 | ||
| Mild intermittent | 24.2 | 24.66 | 22.66 | ||
| Asthma medication use, % | Corticosteroid | 9.7 | 10.8 | 6.7 | 0.20 |
| Non-steroid controllerb | 13.1 | 14.4 | 9.3 | ||
| Short acting bronchodilatorc | 43.3 | 39.9 | 53.3 | ||
| None | 33.9 | 35 | 30.7 |
Percentages are evaluated with respect to the non-missing response, and may not add to 100% due to rounding. Caregiver education, annual house income, caregiver smokes cigarettes and any household member smokes cigarettes have 6, 23, 3 and 2 missing information respectively.
Use of a leukotriene modifier, long-acting bronchodilator, cromolyn, or theophylline, but no use of a corticosteroid.
Use of a short-acting bronchodilator but no use of any controller medication.
3.2. Ambient air pollutants
Levels of all three fractions of PM were higher in the Southwest compared to the Eastside (Table 2). In contrast, O3 levels were minimally higher on the Eastside. Pollutant levels ranged from below to slightly above annual standards, with considerable fluctuation in pollutant levels within and across seasons. Averaged over our entire assessment periods, PM2.5 levels were slightly above the annual NAAQS of 15 μg/m3, at 16.6 μg/m3, while PM10 was well below the annual NAAQS of 50 μg/m3. The maximum 8-hour average value for O3 exceeded 80 ppb on 6 occasions out of a total of 220 measurement-days (3 days in East Detroit; 3 days in SW Detroit), and exceeded 70 ppb on only 13 days. The maximum 1-hour average value for O3 never exceeded 120 ppb. Subsets of this exposure data have been described previously (Keeler et al., 2002). The interquartile ranges for overall measurements were 12.5 μg/m3, 19.1 μg/m3, 14.5 ppb, and 16.0 ppb for PM2.5, PM10, daily O3, and 8-h peak O3, respectively (Lewis et al., 2005).
Table 2.
Ambient pollutant and meteorological measurements in two Detroit communities averaged across 11 seasons (Fall 1999 through Spring 2002)a.
| Mean±sd
|
|||
|---|---|---|---|
| Overall | Eastside | Southwest | |
| O3 daily 24-hour mean (ppb) | 27.3±11.0 | 27.7±11.8 | 26.8±10.2 |
| O3 peak 8-hour mean (ppb) | 41.8±16.0 | 42.5±16.6 | 41.0±15.4 |
| O3 peak 1-hour mean (ppb) | 48.1±18.0 | 48.9±18.8 | 47.3±17.3 |
| PM10 daily mean (μg/m3) | 26.0±14.4 | 23.4±12.9 | 28.5±15.2 |
| PM2.5 daily mean (μg/m3) | 16.6±10.6 | 15.7±10.1 | 17.4±10.9 |
| PM10–2.5b daily mean (μg/m3) | 9.5±6.3 | 7.8±5.1 | 11.1±7.0 |
| Temperature °C (daily maximum) | 17.1±11.0 | 17.3±11.8 | 17.0±10.2 |
| Relative humidity (daily maximum [%]) | 91.3±11.5 | 91.0±11.1 | 91.7±11.8 |
| Temperature °C (daily 24-hour mean) | 12.7±9.3 | 12.5±9.3 | 12.8±9.3 |
| Relative humidity (daily 24-hour mean [%]) | 72.2±13.7 | 71.9±13.6 | 72.6±13.6 |
Ozone was not measured during Fall 1999, Winter 2000, or Winter 2001. Data reflects 8 seasons in which ozone was measured.
Coarse PM fraction was calculated as the difference (PM10–PM2.5).
Overall PM10 was highly correlated with its component fractions, especially with PM2.5 (r=0.92) and to a lesser degree with PM10–2.5 (r=0.76). PM2.5 was only modestly correlated with PM10–2.5 (r=0.43). Very similar values were seen for PM correlations within each community location. Due to these relationships, for assessing the health effects of larger particles, we focused our attention on PM10–2.5 rather than PM10 in order to evaluate distinct size fractions. Additional information on correlations between exposures is included in Supplemental Material.
3.3. Rates of symptoms
Symptom diaries were returned to us by an average of 167 children each season (range 100–237). Most non-completion of diaries was due to participant withdrawal from the study or temporary inability to locate the family to either drop off or collect the diary. No consistent associations were found between participant demographic characteristics and diary non-completion. A total of 22,204 person-days of data were available for analysis out of a target number of person-days based on mean seasonal enrollment of 25,718.
Symptom prevalence reported are the number of person-days in which children reported “yes” to experiencing the symptom divided by the total number of person-days for which the data was available for that symptom. The most commonly reported symptom, cough, is the least specific for asthma, and was reported with 30.1% prevalence (i.e., cough reports occurred in 30.1% of all the days monitored). The average prevalence of reports for more specific symptoms for asthma was 19.4% for wheeze, 18.5% for shortness of breath, and 12.7% for chest tightness, although there was considerable seasonal variation. For all symptoms, highest frequencies were reported in Fall 1999 and a secondary peak in Winter 2001 (shown in Supplemental Fig. 1). The lowest symptom reports were seen in Spring 2002.
3.4. Relationships between air pollution and respiratory symptoms
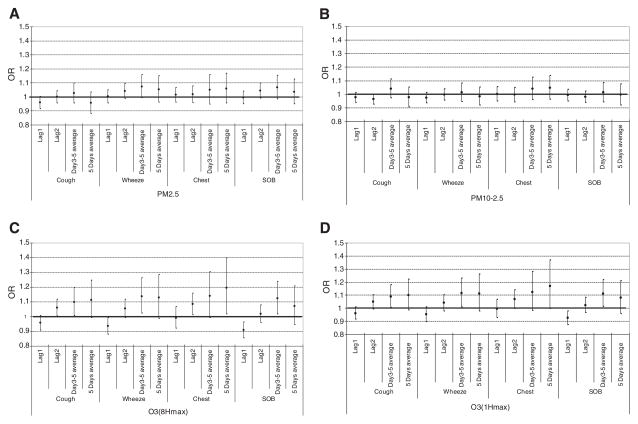
Models examining the association of each pollutant with symptoms for the overall population of asthmatic children are shown in Fig. 1A–D. One-hour peak concentrations of O3 are consistently associated with symptoms, particularly in models involving longer lags, particularly in the 3–5 day range. For example, one interquartile range elevation in O3-1HrPeak during the previous 3–5 days was associated with an odds ratio for wheezing of 1.118 (95% CI: 1.013, 1.233). For PM2.5, PM10–2.5, and O3-8HrPeak, odds ratio estimates generally increase in models with longer lags, though these models do not achieve statistical significance at the p=0.05 level.
Fig. 1.
OR point estimates and 95% confidence intervals of associations of ambient PM2.5, PM10–2.5, PM10, O3-8HrPeak and O3-1HrPeak concentrations with symptoms among children with asthma. 1A. PM2.5 general model. 1B. PM10–2.5 general model. 1C. O3-8HrPeak general model. 1D. O3-1Hr peak general model.
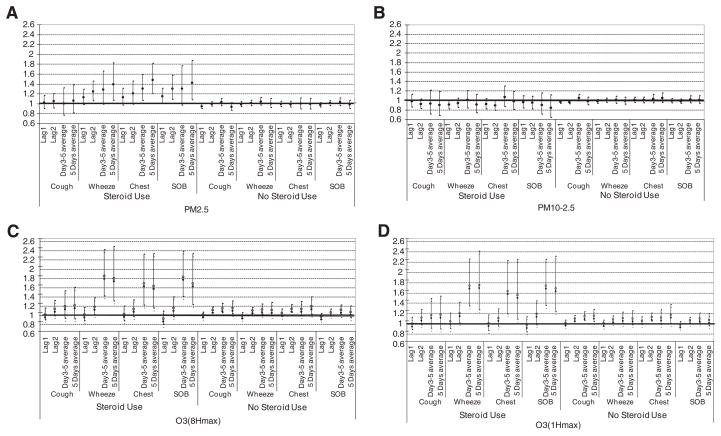
To evaluate whether there were heterogeneous responses to pollution within our population, we utilized models with interaction terms to investigate for possible effect modification. Pollutant-related associations with symptoms were consistently strongest among children taking maintenance corticosteroids (Fig. 2A–D). These relationships were seen for PM2.5, O3-8HrPeak, and O3-1HrPeak, but not for PM10–2.5, and again were seen in the longer lags. While confidence intervals were wide, we observed some of the largest estimated ORs for associations between ozone and lower respiratory tract symptoms (wheeze, chest tightness, and shortness of breath) among children on corticosteroids. In contrast, among children not using steroids, only very limited associations between pollutants and symptoms were observed.
Fig. 2.
OR estimates and 95% confidence intervals of associations of ambient PM2.5, PM10–2.5, O3-8HrPeak and O3-1HrPeak concentrations with symptoms among children with asthma by corticosteroid use. 2A. PM2.5 by steroid use. 2B. PM10–2.5 by steroid use. 2C. O3-8Hr by steroid use. 2D. O3-1Hr by steroid use.
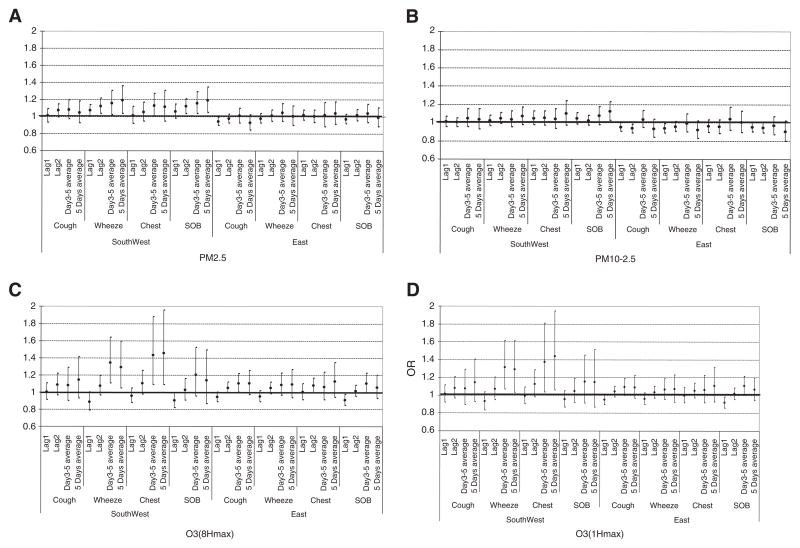
We were also interested in evaluating the potential for different responses by location within Detroit (Fig. 3A–D). Children living in the Southwest area of Detroit had elevated odds of reporting symptoms in response to an interquartile range change in PM2.5, while this association was not seen among children living on the Eastside of Detroit. For example, OR of wheeze associated with elevation in average PM2.5 over the prior 5 days was 1.189 (CI: 1.037, 1.362) in the Southwest, and 1.009 (0.902, 1.128) in the East. Exposure to PM10–2.5 did not produce clear associations with symptoms in either location. Exposure to O3-8HrPeak concentrations was associated with elevated symptom reports in both locations, while associations with O3-1HrPeak concentrations were again strongest among Southwest residents.
Fig. 3.
OR point estimates and 95% confidence intervals of associations of ambient PM2.5, PM10–2.5, O3-8HrPeak and O3-1HrPeak concentrations with symptoms among children with asthma by location. 3A. PM2.5 by location. 3B. PM10–2.5 by location. 3C. O3-8Hr by location. 3D. O3-1Hr by location.
4. Discussion
Ambient PM2.5 and O3 at levels near annual standards were associated with increased respiratory symptoms in this group of urban asthmatic children. Stronger and more frequent associations were seen among those children reported to use corticosteroid medications and those living in Southwest Detroit. Effects associated with PM10–2.5were less consistent than those with PM2.5 and O3.
In this and other studies of our population (Lewis et al., 2005), the adverse impact of pollution on health was seen predominantly amongst children on maintenance corticosteroids. This contrasts with some studies (Delfino et al., 2002; Liu et al., 2009) where effects were seen predominantly in children not using steroids. This difference probably occurs because in some populations, such as ours where steroid use is infrequent, steroid use is a marker for individuals with more severe asthma (Gent et al., 2003), whereas in other populations it may be a marker for better controlled asthma (Delfino et al., 2002). Corticosteroid usage described here represents the participants’ baseline status. While there were relatively low rates of corticosteroid use in our population, resulting in fairly wide 95% confidence intervals when evaluating this subpopulation, we obtained robust estimates by using interaction terms in our models which allows consideration of data from all children.
The association of symptoms with PM2.5 varied by location within the city, with stronger associations observed in the Southwest community. Similar differential effects of PM2.5 by location within Detroit were identified in a study of air pollution and cardiovascular outcomes among Detroit adults conducted 2002–2003, with stronger effects also observed among Southwest residents (Dvonch et al., 2009; Kannan et al., 2010). The local pollutant emission sources differ in each of the two Detroit communities studied, suggesting to us that the stronger associations observed in the Southwest community may reflect differences in PM composition. Specifically, while industrial and traffic sources of pollutants are present in both communities, heavy industrial sites including iron/steel manufacturing, coke ovens, chemical plants, refineries, sewage sludge incineration and coal-fired utilities are concentrated within 5 km immediately upwind of the Southwest neighborhood. Southwest Detroit also contains the Ambassador Bridge, the largest international border crossing for truck traffic between the U.S. and Canada, carrying over 3.5 million trucks per year. At the time of this study, no direct highway link between the Bridge and the interstate highway system existed, so trucks entering and exiting the Bridge had to travel on community surface streets to connect to highways. The result has been long lines of trucks idling at traffic lights along busy city streets. In a related source apportionment analysis conducted as part of our study, one third of the PM2.5 in this Southwest Detroit community was from motor vehicle emissions (Hammond et al., 2008). The exposures experienced in Southwest Detroit likely reflect a combination of regional and local sources, whereas Eastside likely represents a greater percentage of regional sources. This can be evaluated with source apportionment-based health effect analysis, one of our next goals.
Potential alternative explanations for the observed differences by location include the possibility of enhanced precision of measurements at the SW location or enhanced susceptibility of the populations living in these areas. However, we think these are less likely to explain the observed findings. While it is true that the area of SW is smaller than the Eastside so, on average, people in SW live closer to monitors than in the East, there are other factors that may be making measures in SW less precise. There are point sources with temporally variable emissions that are closer to residences in SW than E, which likely sets up steeper and more variable mass gradients. Also, there is more inherent variability of mobile sources due to traffic patterns in SW, notably trucks idling on surface streets while in a queue waiting to enter the nearby bridge to Canada. Together, these competing factors make it difficult to determine whether the precision of the PM2.5 mass estimates are more precise in one community or another, though our general impression is that the errors, on average, are similar.
We explored the possibility that differing characteristics of the populations of the two communities might explain the enhanced risk of symptoms associated with pollution in Southwest as compared to Eastside. The overall characteristics of the two communities were quite similar, with a notable exception being differences in the ethnic/racial background of residents. We conducted an analysis restricted to African Americans, and observed continued differences in symptom risk between Southwest and Eastside residents. We also conducted an analysis restricted to Southwest residents, and saw statistically significant effects among both children reporting African-American and non-African American heritage.
We further explored the possibility that the observed associations with location and corticosteroid use were due to a subgroup with both characteristics. While we could not obtain stable estimates of a two-way interaction between corticosteroid use and location of residence due to the small numbers, if anything, a slightly lower proportion of Southwest residents took corticosteroids (6.7%) relative to Eastside residents (10.8%), which would suggest that increased corticosteroid use does not explain the increased vulnerability of Southwest residents. As an alternative, we ran models looking at effect modification by corticosteroid use, restricting to Eastside residents only and found very similar patterns to the overall analysis. Point estimates were nearly identical to the unrestricted models, though only 2 of 7 PM2.5 models retained statistical significance at alpha=0.05 due to slightly larger confidence intervals associated with smaller sample size. There was still no significance among non-corticosteroid users. The associations for ozone and respiratory symptoms in the later lags remained significant for steroid users in the East-restricted models. We are reassured by the consistency of results and feel that the restricted analyses support that there is a vulnerability of the children who use corticosteroids which is different than that seen among SW residents. We interpret these supplemental analyses as supporting our impression that observed differences in location are less likely to be an effect associated with the surrogate indicator of race/ethnicity, differences in corticosteroid use patterns, and more likely related to differences in pollution composition or exposure patterns.
Several challenges of the study involved assessing symptoms using handwritten diaries. We had difficulty in obtaining completed data records despite concerted efforts to maintain contact with families and provide community-acceptable incentives. We performed several analyses to examine whether we could detect non-randomness of the missing data or associations between missing data and asthma severity or covariates, and none were found. We cannot, however, completely exclude some undetected relationship between completeness of data and susceptibility to air pollution, which could have introduced bias to these analyses. Another limitation of symptomdiaries is that caregivers and children may not have filled in the diary together on a daily basis as instructed, potentially introducing measurement error. Fortunately, despite issues inherent in diaries, good correlation is seen between asthma activity assessed by symptomdiaries and by other methods (Gold et al., 1989; Hensley et al., 2003).
Although many challenges with recruitment and retention exist for a study of this length and intensity (Eggleston et al., 2005), the CBPR approach and guidance from our community partners was instrumental in addressing these challenges in a city where many families struggle with financial and social stressors. An intense effort was made to maintain contact and involvement with enrolled families. Retention rates and degree of data missingness are similar to other reports in the literature (Eggleston et al., 2005; Mortimer et al., 2002).
Several elements of the study design strengthen our results. Among them are daily repeated measures of both exposure and symptom outcome across multiple seasons. Exposure data was collected at two neighborhood-level monitoring sites, which allowed finer spatial resolution than is often available.
While size of the increased odds of symptoms is relatively small, given the large number of people in the U.S. with asthma, small increases in symptom frequency have important potential public health consequences. In addition to the direct physical impact on an asthmatic child, active asthma symptoms often have important indirect effects, such as school absenteeism and loss of workdays for caregivers.
As more information about the short-term respiratory effects of air pollution becomes known, physicians can address the role of ambient air pollution as part of their asthma education with patients. Little data is available addressing which medical treatments (if any) can protect against the negative impacts of air pollution. Therefore, educational messages to susceptible patients focus on limiting outdoor activity, particularly vigorous activity, on days when ambient air quality is poor.
While average levels of PM2.5 were above the annual U.S. EPA NAAQS in effect at the time of the study, average ambient levels of PM10 (daily) and O3 (8-hour maximum) in our study were near or below the NAAQS. Ozone standards were tightened in 2008, which should be beneficial for health, though only if standards are enforced and attained. Many regions of the country remain out of attainment for PM2.5 and O3 standards, and our data suggests that vulnerable populations may be susceptible to adverse health effects at levels even below current standards. Emissions from motor vehicle traffic in Detroit are substantial due to limited public transportation and high commercial truck traffic volume on interstate highways and through the international border crossing with Canada. Efforts to control emissions and develop transportation infrastructure should take into account health impacts in nearby communities.
In conclusion, ambient PM2.5, PM10, and O3 are all significant triggers of asthma symptoms, particularly among children in our cohort with more severe asthma as indicated by corticosteroid therapy and among residents in Southwest Detroit. Our work shows that vulnerable subpopulations asthmatic children experience an increase in respiratory symptoms in association with prevalent fluctuations of these pollutants. This increased health impact stresses the need for enhanced understanding of mechanisms of vulnerability, community-level air quality monitoring, and ongoing refinement and enforcement of national air quality standards.
Supplementary Material
HIGHLIGHTS.
Children with asthma may have heterogeneous responses to air pollution.
We examined relationships between particulate matter, ozone, and asthma symptoms.
Impacts of air pollution were stronger among children with more severe asthma.
Effects of air pollution also differed by location within the City of Detroit.
Acknowledgments
Community Action Against Asthma (CAAA) is a community-based participatory research partnership that, at the time of this project, consisted of the following organizations: University of Michigan School of Public Health, Detroit Hispanic Development Corporation, University of Michigan School of Medicine, Friends of Parkside, Detroit Department of Health and Wellness Promotion, Kettering/Butzel Health Initiative, Michigan Department of Agriculture — Plant and Pest Management Division, Latino Family Services, United Housing Coalition, Butzel Family Center, Warren/Conner Development Coalition, Community Health and Social Services Center Inc., Henry Ford Health System, Detroiters Working for Environmental Justice. CAAA is an affiliated project of the Detroit Community-Academic Urban Research Center.
Abbreviations
- CAAA
Community Action Against Asthma
- CBPR
community-based participatory research
- NAAQS
National Ambient Air Quality Standards
- O3
Ozone
- PM2.5
Particulate matter less than 2.5 μm aerodynamic diameter
- PM10
Particulate matter less than 10 μm aerodynamic diameter
- PM10–2.5
Particulate matter between 2.5 and 10 μm aerodynamic diameter
- U.S. EPA
United States Environmental Protection Agency
- 1HrPeak
Ozone 1-hour peak
- 8HrPeak
Ozone 8-hour peak
Appendix A. Supplementary data
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.scitotenv.2012.11.070.
Footnotes
Sources of support: This work was supported by the United States Environmental Protection Agency (R826710), the National Institute of Environmental Health Sciences (K23–ES013242, P01-ES09589, R01-ES010688, and R01-ES016932), and the Robert Wood Johnson Health & Society Scholars Program (M. O’Neill).
References
- Brook RD, Rajagopalan S, Pope CA, III, Brook JR, Bhatnagar A, Diez-Roux AV, et al. Particulate matter air pollution and cardiovascular disease: an update to the scientific statement from the American Heart Association. Circulation. 2010;121(21):2331–78. doi: 10.1161/CIR.0b013e3181dbece1. [DOI] [PubMed] [Google Scholar]
- Dales R, Chen L, Frescura AM, Liu L, Villeneuve PJ. Acute effects of outdoor air pollution on forced expiratory volume in 1 s: a panel study of schoolchildren with asthma. Eur Respir J. 2009;34(2):316–23. doi: 10.1183/09031936.00138908. [DOI] [PubMed] [Google Scholar]
- Delfino RJ, Zeiger RS, Seltzer JM, Street DH, McLaren CE. Association of asthma symptoms with peak particulate air pollution and effect modification by anti-inflammatory medication use. Environ Health Perspect. 2002;110(10):607–17. doi: 10.1289/ehp.021100607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennekamp M, Akram M, Abramson MJ, Tonkin A, Sim MR, Fridman M, et al. Outdoor air pollution as a trigger for out-of-hospital cardiac arrests. Epidemiology. 2010;21(4):494–500. doi: 10.1097/EDE.0b013e3181e093db. [DOI] [PubMed] [Google Scholar]
- Dominici F, McDermott A, Daniels M, Zeger SL, Samet JM. Revised analyses of the National Morbidity, Mortality, and Air Pollution Study: mortality among residents of 90 cities. J Toxicol Environ Health A. 2005;68(13–14):1071–92. doi: 10.1080/15287390590935932. [DOI] [PubMed] [Google Scholar]
- Dominici F, Peng RD, Bell ML, Pham L, McDermott A, Zeger SL, et al. Fine particulate air pollution and hospital admission for cardiovascular and respiratory diseases. JAMA. 2006;295(10):1127–34. doi: 10.1001/jama.295.10.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dvonch JT, Kannan S, Schulz AJ, Keeler GJ, Mentz G, House J, et al. Acute effects of ambient particulate matter on blood pressure: differential effects across urban communities. Hypertension. 2009;53(5):853–9. doi: 10.1161/HYPERTENSIONAHA.108.123877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgren KK, Parker EA, Israel BA, Lewis TC, Salinas MA, Robins TG, et al. Conducting a health education intervention and epidemiological research project involving community members and community partner organizations: the Community Action Against Asthma Project. Health Promot Pract. 2005;6(3):263–9. doi: 10.1177/1524839903260696. [DOI] [PubMed] [Google Scholar]
- Eggleston P, Diette G, Lipsett M, Lewis TC, Tager IB, McConnell R, et al. Lessons learned from the study of childhood asthma from the Centers for Children’s Environmental Health and Disease Prevention Research. Environ Health Perspect. 2005;113(10):1430–6. doi: 10.1289/ehp.7671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauderman WJ, Avol E, Gilliland F, Vora H, Thomas D, Berhane K, et al. The effect of air pollution on lung development from 10 to 18 years of age 10. N Engl J Med. 2004;351(11):1057–67. doi: 10.1056/NEJMoa040610. [DOI] [PubMed] [Google Scholar]
- Gauderman WJ, Avol E, Lurmann F, Kuenzili N, Gilliland F, Peters J, et al. Childhood asthma and exposure to traffic and nitrogen dioxide. Epidemiology. 2005;16(6):737–43. doi: 10.1097/01.ede.0000181308.51440.75. [DOI] [PubMed] [Google Scholar]
- Gent JF, Triche EW, Holford TR, Belanger K, Bracken MB, Beckett WS, et al. Association of low-level ozone and fine particles with respiratory symptoms in children with asthma. JAMA. 2003;290(14):1859–67. doi: 10.1001/jama.290.14.1859. [DOI] [PubMed] [Google Scholar]
- Gold DR, Weiss ST, Tager IB, Segal MR, Speizer FE. Comparison of questionnaire and diary methods in acute childhood respiratory illness surveillance. Am Rev Respir Dis. 1989;139(3):847–9. doi: 10.1164/ajrccm/139.3.847. [DOI] [PubMed] [Google Scholar]
- Gold DR, Wright RJ. Population disparities in asthma. Annu Rev Public Health. 2005;26:89–113. doi: 10.1146/annurev.publhealth.26.021304.144528. [DOI] [PubMed] [Google Scholar]
- Hammond DM, Dvonch JT, Keeler GJ, Parker EA, Kamal AS, Barres JA, et al. Sources of ambient fine particulate matter at two community sites in Detroit, Michigan. Atmos Environ. 2008;42:720–32. [Google Scholar]
- He QQ, Wong TW, Du L, Jiang ZQ, Gao Y, Qiu H, et al. Effects of ambient air pollution on lung function growth in Chinese schoolchildren. Respir Med. 2010;104(10):1512–20. doi: 10.1016/j.rmed.2010.04.016. [DOI] [PubMed] [Google Scholar]
- Hensley MJ, Chalmers A, Clover K, Gibson PG, Toneguzzi R, Lewis PR. Symptoms of asthma: comparison of a parent-completed retrospective questionnaire with a prospective daily symptom diary. Pediatr Pulmonol. 2003;36(6):509–13. doi: 10.1002/ppul.10360. [DOI] [PubMed] [Google Scholar]
- Israel BA, Parker EA, Rowe Z, Salvatore A, Minkler M, López J, et al. Community-based participatory research: lessons learned from the Centers for Children’s Environmental Health and Disease Prevention Research. Environ Health Perspect. 2005;113(10):1463–71. doi: 10.1289/ehp.7675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Israel BA, Schulz AJ, Parker EA, Becker AB, Allen AJ, Guzman JR. Critical issues in developing and following community-based participatory research principles. In: Minkler M, Wallerstein N, editors. Community-Based Participatory Research for Health. 2. San Francisco, CA: Jossey-Bass; 2008. pp. 47–66. [Google Scholar]
- Jerrett M, Burnett RT, Ma R, Pope CA, III, Krewski D, Newbold KB, et al. Spatial analysis of air pollution and mortality in Los Angeles. Epidemiology. 2005;16:727–36. doi: 10.1097/01.ede.0000181630.15826.7d. [DOI] [PubMed] [Google Scholar]
- Kannan S, Dvonch T, Schulz AJ, Israel BA, Mentz G, House J, et al. Exposure to fine particulate matter and acute effects on blood pressure: effect modification by measures of obesity and location. J Epidemiol Community Health. 2010;64(1):68–74. doi: 10.1136/jech.2008.081836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keeler GJ, Dvonch JT, Yip F, Parker EA, Israel BA, Marsik FJ, et al. Assessment of personal and community-level exposures to particulate matter among children with asthma in Detroit, Michigan, as part of Community Action Against Asthma (CAAA) Environ Health Perspect. 2002;110(Suppl 2):173–81. doi: 10.1289/ehp.02110s2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis TC, Robins TG, Dvonch JT, Keeler GJ, Yip FY, Mentz GB, et al. Air pollution-associated changes in lung function among asthmatic children in Detroit. Environ Health Perspect. 2005;113(8):1068–75. doi: 10.1289/ehp.7533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis TC, Robins TG, Joseph CLM, Parker EA, Israel BA, Rowe Z, et al. Identification of gaps in the diagnosis and treatment of childhood asthma using a community-based participatory research approach. J Urban Health. 2004;81(3):472–88. doi: 10.1093/jurban/jth131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Poon R, Chen L, Frescura AM, Montuschi P, Ciabattoni G, et al. Acute effects of air pollution on pulmonary function, airway inflammation, and oxidative stress in asthmatic children. Environ Health Perspect. 2009;117(4):668–74. doi: 10.1289/ehp11813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann JK, Balmes JR, Bruckner TA, Mortimer KM, Margolis HG, Pratt B, et al. Short-term effects of air pollution on wheeze in asthmatic children in Fresno, California. Environ Health Perspect. 2010;118(10):1497–502. doi: 10.1289/ehp.0901292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortimer KM, Neas LM, Dockery DW, Redline S, Tager IB. The effect of air pollution on inner-city children with asthma. Eur Respir J. 2002;19(4):699–705. doi: 10.1183/09031936.02.00247102. [DOI] [PubMed] [Google Scholar]
- Mortimer KM, Tager IB, Dockery DW, Neas LM, Redline S. The effect of ozone on inner-city children with asthma: identification of susceptible subgroups. Am J Respir Crit Care Med. 2000;162(5):1838–45. doi: 10.1164/ajrccm.162.5.9908113. [DOI] [PubMed] [Google Scholar]
- Ostro B, Lipsett M, Mann J, Braxton-Owens H, White M. Air pollution and exacerbation of asthma in African-American Children in Los Angeles. Epidemiology. 2001;12(2):200–8. doi: 10.1097/00001648-200103000-00012. [DOI] [PubMed] [Google Scholar]
- Parker EA, Israel BA, Brakefield-Caldwell W, Keeler GJ, Lewis TC, Ramirez E, et al. Community Action Against Asthma: examining the partnership process of a community-based participatory research project. J Gen Intern Med. 2003;18(7):558–67. doi: 10.1046/j.1525-1497.2003.20322.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker EA, Israel BA, Robins TG, Mentz G, Lin X, Brakefield-Caldwell W, et al. Evaluation of Community Action Against Asthma: a community health worker intervention to improve children’s asthma-related health by reducing household environmental triggers for asthma. Health Educ Behav. 2008;35(3):376–95. doi: 10.1177/1090198106290622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Q, Hong X, Wold LE. Cardiovascular effects of ambient particulate air pollution exposure. Circulation. 2010;121(25):2755–65. doi: 10.1161/CIRCULATIONAHA.109.893461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- United States Environmental Protection Agency. Review of the National Ambient Air Quality Standards for Particulate Matter: policy assessment of scientific and technical information. Research Triangle Park, NC: Office of Air Quality Planning and Standards; 2005a. OAQPS Staff Paper EPA-452/D-05-005a. [Google Scholar]
- United States Environmental Protection Agency. U.S. EPA. Office of Air Quality Planning and Standards. [accessed Nov 18, 2009];Green book nonattainment areas for criteria pollutants. 2005b [Available: http://www.epa.gov/oar/oaqps/greenbk/
- United States Environmental Protection Agency. U.S. EPA. Office of Air and Radiation, Air Quality System. Exceedances of air quality standards. 2010 [Google Scholar]
- Weinmayr G, Romeo E, De Sario M, Weiland SK, Forastiere F. Short-term effects of PM10 and NO2 on respiratory health among children with asthma or asthma-like symptoms: a systematic review and meta-analysis. Environ Health Perspect. 2010;118(4):449–57. doi: 10.1289/ehp.0900844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yip FY, Keeler GJ, Dvonch JT, Robins TG, Israel BA, Brakefield-Caldwell W. Personal exposures to particulate matter among children with asthma in Detroit, Michigan. Atmos Environ. 2004;38:5227–36. doi: 10.1289/ehp.02110s2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.