Figure 1. Schematic of oxygen-dependent HIF-1α regulation.
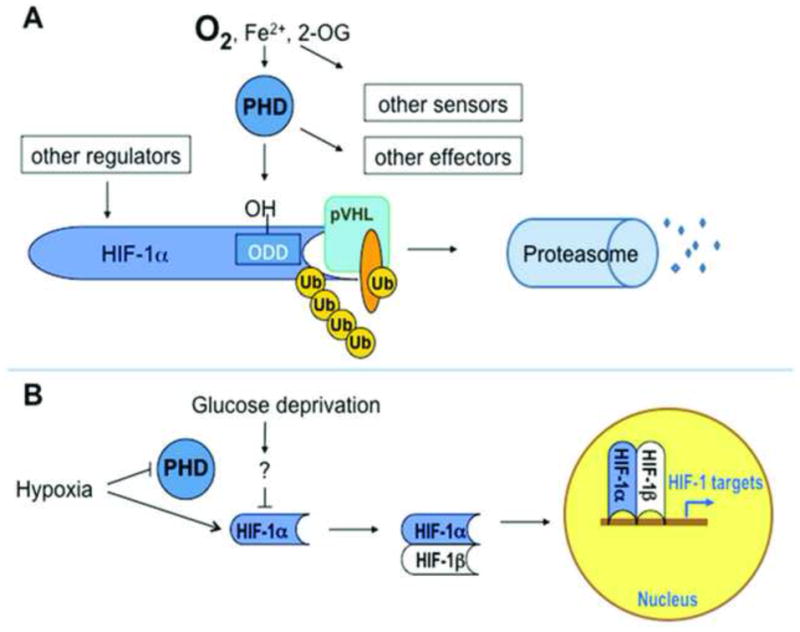
A. Under normoxic conditions, PHDs hydroxylate P564 on HIF-1α, allowing it to be recognized by the E3 ubiquitin ligase von Hippel-Lindau protein (VHL), ubiquitinated, and targeted for proteasomal degradation. As members of a large family of iron- and 2-oxoglutarate dependent dioxygenases, PHDs integrate multiple signals of metabolic homeostasis, and are one of many such sensors; further, PHDs have HIF-independent substrates, and HIF protein levels and transcriptional activity are regulated in many PHD-independent ways. B. Under hypoxia, PHDs are inhibited, allowing HIF-1α to elude degradation, dimerize with its β partner in the nucleus, bind transcriptional coactivators and hypoxia response elements in promoter regions of target genes, and enhance transcription rates. Glucose deprivation has been reported to decrease hypoxic stabilization of HIF-1α; the mechanisms by which this occurs are unclear.
