Figure 1.
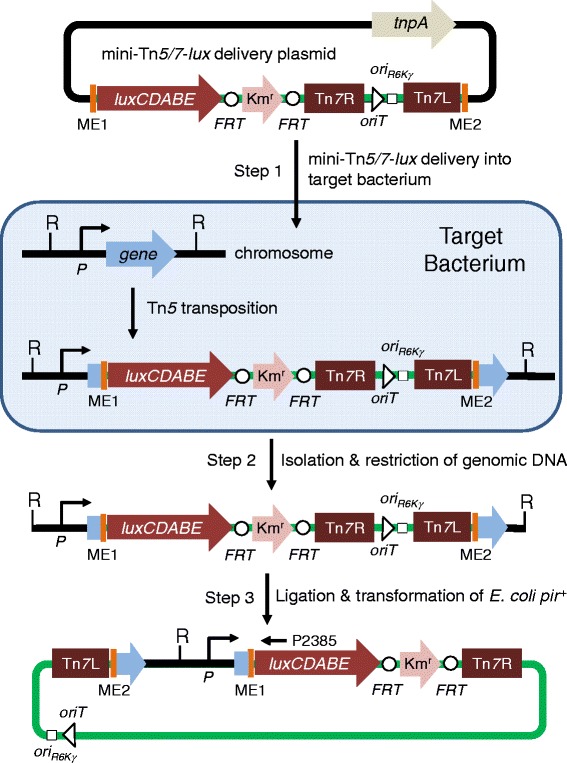
Promoter identification and capture using mini-Tn 5/7-lux elements. Step 1 involves random Tn5 transposition into the chromosome of the bacterial host of interest (target bacterium) after conjugal transfer of the mini-Tn5/7-lux delivery plasmid. This plasmid also contains the Tn5 transposase encoding tnpA gene. TnpA acts on the Tn5 mosaic ends (ME), which results in random mini-Tn5/7-lux transposition into the bacterial chromosome, including insertion in a gene where the Photorhabdus luminescens luxCDABE operon is transcribed from the gene’s promoter (P). The mini-Tn7 element, i.e. sequences flanked by the Tn7 left (Tn7L) and right (Tn7R) ends, located on the mini-Tn5/7-lux delivery plasmid does not transpose in this step because the delivery plasmid does not encode Tn7 transposase. Mini-Tn5/7-lux chromosomal insertion is stable because the chromosomally-integrated elements do neither encode Tn5 nor Tn7 transposase. In step 2, chromosomal DNA of in this example kanamycin resistance (Kmr) and light-emitting transformants is isolated and digested with a restriction enzyme (R) that does not cleave within the transposed element. In step 3, chromosomal DNA fragments are religated and a plasmid containing the R6K origin of replication (ori R6K) and origin of conjugal transfer (oriT) is recovered by transformation of a pir + E. coli host and selecting Kmr transformants. Sequencing of plasmid DNA with a luxC-specific primer (P2385) will reveal the transposon insertion site and putative promoter sequences. The Kmr selection marker contained on the chromosomally integrated mini-Tn7-lux elements is flanked by Flp recombinase target (FRT) sites for optional marker excision with Saccharomyces cerevisiae Flp recombinase.
