Figure 1.
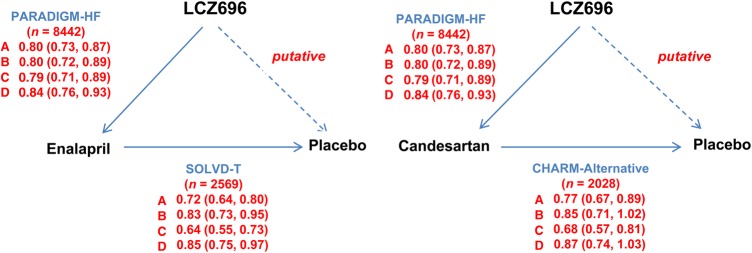
Schematic of the trials and comparisons used in the putative placebo analysis. SOLVD-T = treatment arm of the Studies Of Left Ventricular Dysfunction (comparing enalapril with placebo). CHARM-Alternative = Candesartan in Heart failure: Assessment of Reduction in Mortality and morbidity-Alternative trial (comparing candesartan with placebo). PARADIGM-HF = Prospective comparison of Angiotensin Receptor Neprilysin inhibitor (ARNI) with ACEI to Determine Impact on Global Mortality and morbidity in Heart Failure (comparing LCZ696 with enalapril). For enalapril, solid arrows indicate directly performed comparison in the trials specified (direction of arrow indicates comparison of experimental treatment to the reference treatment); the interrupted arrow indicates the indirect, putative, placebo comparison (direction of arrow indicates comparison of experimental treatment to the putative placebo). For candesartan, the figure structure is the same except that it is assumed that the comparison in PARADIGM-HF was LCZ696 vs. candesartan (as opposed to enalapril in reality). The hazard ratios shown are those measured in the trials specified; the indirectly calculated hazard ratios for LCZ696 against placebo are shown in Table 3. (a) Cardiovascular death or heart failure hospitalization, (b) cardiovascular death, (c) heart failure hospitalization, and (d) all-cause mortality.
