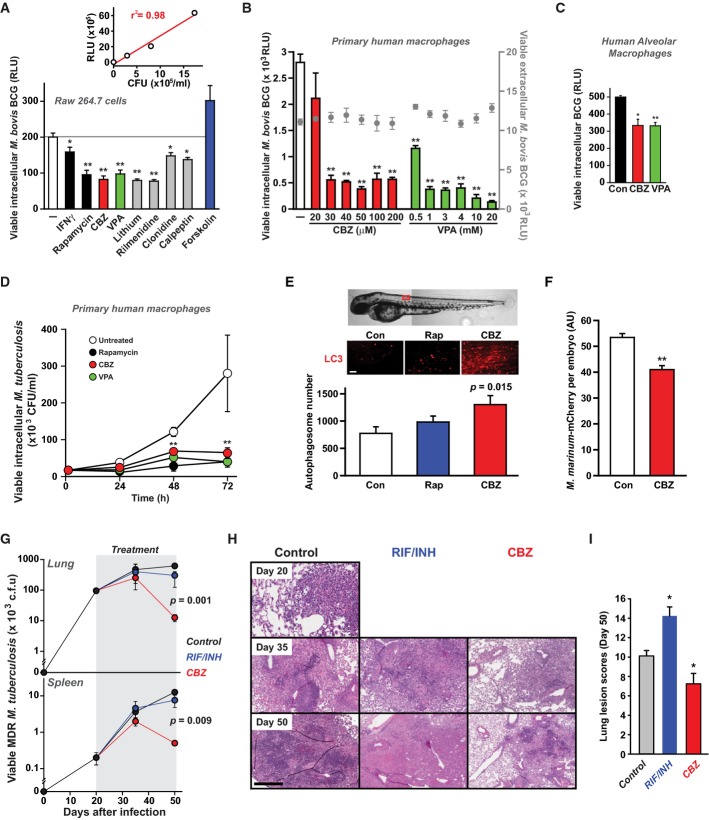
Figure 1.
Anticonvulsants stimulate killing of mycobacteria in vitro
- Screening drugs for effects on survival of intracellular mycobacteria in macrophages. RAW 264.7 cells were infected with a luminescent strain of M. bovis BCG (BCG-lux) for 1 h, washed and treated for 24 h with vehicle alone (white), known autophagy enhancers (interferon-γ (IFNγ), 200 ng/ml and rapamycin, 200 nM; black), known mTOR-independent autophagy inhibitor (forskolin; 24 μM; blue), carbamazepine (CBZ, 50 μM; red), valproic acid (VPA, 3 mM; green) and other examples of hits from a large screen of compounds enhancing intracellular killing of mycobacteria (lithium, 10 mM; rilmenidine, 1 μM; clonidine, 1 μM; calpeptin, 50 μM; grey). P-values, unpaired Student's t-test (n ≥ 6) (compared to vehicle alone): IFNγ 0.03; rapamycin 0.003, CBZ 0.001, VPA 0.001, lithium 0.001, rilmenidine 0.001; clonidine 0.02; calpeptin 0.01. Inset: Correlation between measurements of colony-forming units (CFU) and luminescence (RLU) for cultures of M. bovis BCG-lux as previously described (Kampmann et al, 2000).
- Effects on intracellular survival of M. bovis BCG-lux of treatment with varying concentrations of CBZ (red) or VPA (green). P-values, unpaired Student's t-test (n = 6) (compared to vehicle alone) for CBZ 30 μM: 9 × 10−7, 40 μM: 1.9 × 10−9, 50 μM: 1.3 × 10−9, 100 μM: 1.6 × 10−8, 200 μM: 2.7 × 10−9, VPA 0.5 mM: 6.9 × 10−8, 1 mM: 1.6 × 10−9, 3 mM: 4.3 × 10−10, 4 mM: 4.2 × 10−9, 10 mM: 1.1 × 10−9, 20 mM: 4.2 × 10−10. These compounds had no effect on cell-free mycobacterial viability (grey circles).
- Anticonvulsants enhance intracellular killing of mycobacteria within human alveolar macrophages. Alveolar macrophages, obtained from the broncho-alveolar lavage fluid of three individuals, were infected with M. bovis BCG-lux and then treated with carbamazepine (CBZ, 50 μM; red), valproic acid (VPA, 3 mM; green) or vehicle alone (Con; black). Viable intracellular mycobacteria were determined after 24 h of treatment. Unpaired Student's t-test (n = 3) (compared to vehicle alone): CBZ 0.011; VPA 0.0009.
- Enhanced killing of intracellular M. tuberculosis (H37Rv) within primary human macrophages by treatment with CBZ (50 μM; red), VPA (3 mM; green) and rapamycin (200 nM; black), compared to control (vehicle alone; white). Unpaired Student's t-test (n = 6) (compared to vehicle alone): rapamycin 48 h 0.00002, rapamycin 72 h 0.00005, CBZ 48 h 0.00021, CBZ 72 h 0.00022, VPA 48 h 0.0002, VPA 72 h 0.00049.
- In vivo induction of autophagy by CBZ (50 μM; red) and rapamycin (RAP; 1 μM; blue) compared to vehicle control (white) monitored in zebrafish expressing fluorescent ATG8 co-treated with chloroquine to delay degradation of autophagosomes.
- Wild-type zebrafish were injected with a red fluorescently tagged M. marinum strain M into the yolk sac circulation valley at 28 hpf. Larvae were imaged at 120 hpf by confocal microscopy and the total mycobacteria-associated fluorescence quantified using Volocity® software. Data expressed as mean ± SEM (n ≥ 13 fish performed as 3 independent experiments). P-values, unpaired Student's t-test (compared to vehicle alone): 0.0035.
- Mice infected via aerosol with a highly virulent clinical strain of multidrug-resistant M. tuberculosis CSU 87 were treated from day 20 post-infection with carbamazepine (CBZ, 50 μg/kg i.p. daily), rifampicin/isoniazid (RIF/INH) or vehicle control (n = 5 per time point per group). CBZ treatment for 30 days resulted in (G) significantly less viable bacteria detected in lung and spleen, (H) reduced inflammatory pulmonary infiltrates compared to RIF/INH-treated or control animals and (I) decreased lung lesion scores. Unpaired Student's t-test (n = 5) (compared to vehicle alone): RIF/INH 0.007; CBZ 0.037.
Data information: *P < 0.05; **P < 0.005.