Figure 8.
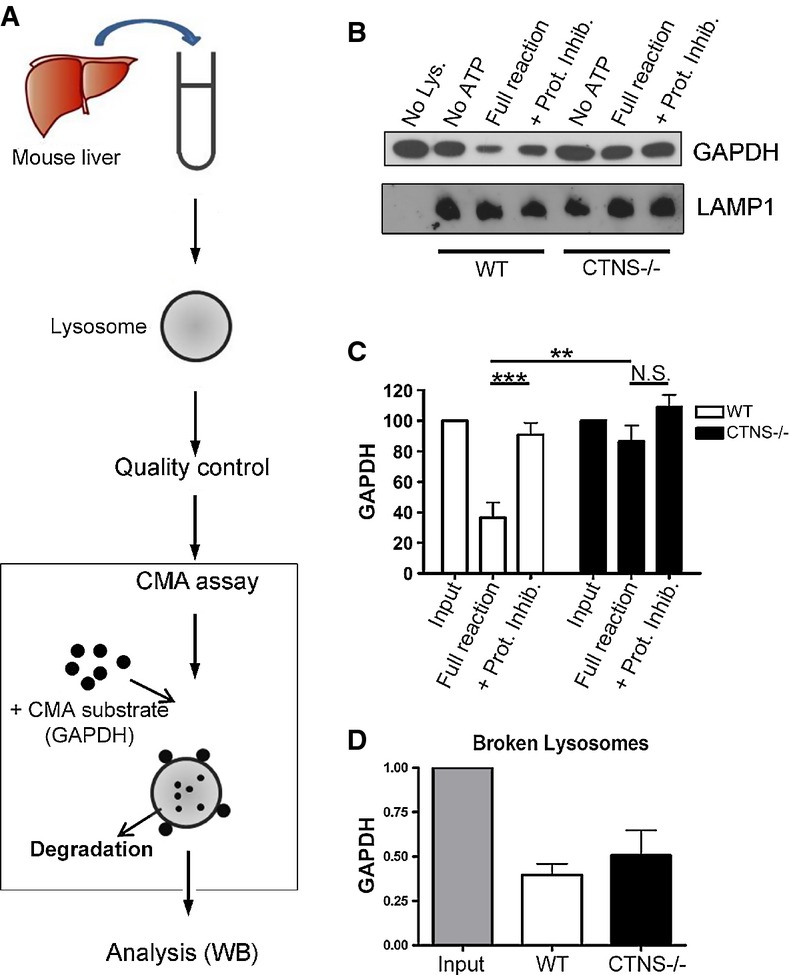
- Schematic representation of lysosomal isolation and in vitroCMA assay [adapted from Kaushik and Cuervo (2008)].
- Lysosomes were isolated from livers of starved wild-type (WT) and Ctns−/− mice as described in Supplementary Materials and Methods and incubated at 37°C for 30 min with the CMA substrate GAPDH, in the presence or absence of ATP (necessary for CMA) and protease inhibitors (Prot. Inhib.). A fraction of the CMA reactions was then mixed with sample buffer and boiled at 95°C for 5 min, followed by SDS–PAGE and GAPDH and LAMP1 immunoblotting.
- Quantitative densitometry analysis of CMA activity performed in independent experiments using lysosomes isolated from a total of 11 WT and 10 Ctns−/− mice. Results are mean ± SEM. ***P = 0.0005; **P = 0.0041; N.S., not significant (unpaired t-test).
- The ability of lysosomal proteases to mediate GAPDH degradation was assessed by incubating Triton X-100-treated WT and Ctns−/− lysosomes with GAPDH for 30 min in acidic reaction buffer and compared with input (reaction with no lysosomes). Quantitative densitometry analysis of independent reactions performed with lysosomes from a total of 9 WT and 9 Ctns−/− mice shows no significant difference in protease activity. Results are mean ± SEM.
Source data are available online for this figure.
