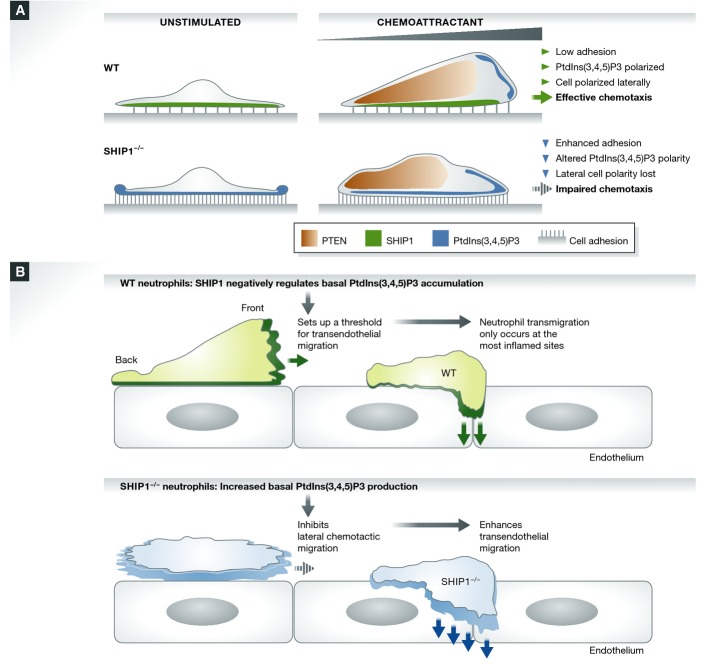
Figure 2.
The role of SHIP1 and PTEN in neutrophil chemotactic migration
(A) SHIP1 and PTEN act via different receptor-regulated processes to control spatial accumulation of PtdIns(3,4,5)P3 and establish a proper anterior–posterior PtdIns(3,4,5)P3 compass. SHIP1 acts as a negative regulator of integrin-mediated cell adhesion in neutrophils. In wild-type neutrophils, integrin-mediated cell adhesion results in PtdIns(3,4,5)P3 production at the sites of cell adhesion. Concurrently, SHIP1 at the cell–substratum interface is engaged, phosphorylated, and activated. This activity is crucial for dephosphorylating the PtdIns(3,4,5)P3 formed during cell adhesion. With the combined actions of both SHIP1 and PTEN, PtdIns(3,4,5)P3 polarity is maintained at the leading edge, neutrophils polarize, and there is effective cell migration. PTEN is localized to the rear end of a migrating cell to facilitate the accumulation of PtdIns(3,4,5)P3 at the anterior end, and SHIP1 is active at the cell–substratum interface to abolish the PtdIns(3,4,5)P3 gradient being formed by integrin activation. With loss of SHIP1, adhesion-mediated PtdIns(3,4,5)P3 formation is uncontrolled, resulting in the formation of “top-down” PtdIns(3,4,5)P3 polarity. Increased PtdIns(3,4,5)P3 levels enhance cell adhesion. This leads to activation of various PtdIns(3,4,5)P3 effector proteins and consequently results in impaired chemotaxis. (B) SHIP1 plays a critical role in neutrophil transendothelial migration by balancing the lateral and dorsal–ventral polarity of transmigrating neutrophils.