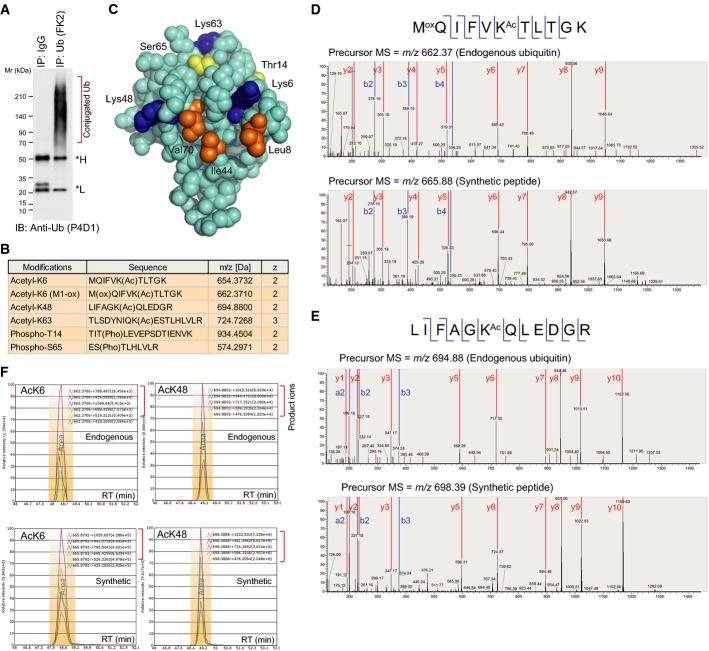
Figure 1.
- Preparation of conjugated ubiquitin from cell lines. Cell lysates were subjected to immunoprecipitation using an anti-Ub (FK2) antibody. Asterisks indicate the antibody heavy and light chains.
- Summary of the identified PTMs modifying ubiquitin. More details are provided in Supplementary Fig S2.
- Structural view of the ubiquitin modification sites. The images were drawn from PDB 1F9J. Acetylation sites, blue. Phosphorylation sites, yellow. Hydrophobic patch, orange.
- MS/MS spectra identifying acetylation on endogenous ubiquitin at K6 (D) and K48 (E). For each panel, the upper spectra are obtained from cell-derived ubiquitin prepared in (A), and the lower spectra are from synthetic, isotopically labeled AQUA peptides. Identified b and y fragment ions are shown.
- Sample-derived peptides containing acetyllysine at either K6 (left) or K48 (right), and the synthetic, isotopically labeled counterparts co-eluted at the same retention times. The detected fragment ions are listed.
Source data are available online for this figure.