Abstract
Purpose
To design a proof-of-concept study to assess the effect of lacrimal nerve stimulation (LNS) with an implantable pulse generator (IPG) to increase aqueous tear production.
Methods
Experimental animal study design of six Dutch Belted rabbits. Ultra high-resolution optical coherence tomography (UHR-OCT) quantified tear production by measuring the baseline tear volume of each rabbit’s right and left eye. A neurostimulator was implanted adjacent to the right lacrimal nerve. After two minutes of LNS (100 μs, 1.6 mAmp, 20 Hz, 5–8 volts), the tear volumes were measured with UHR-OCT. The change in tear volume was quantified and compared to the non-stimulated left eye. Three rabbits underwent chronic LNS (100 μS, 1.6 mAmp, 10 Hz, 2 volts) and their lacrimal glands were harvested for histopathologic analysis.
Results
UHR-OCT imaging of the right eyes tear volume showed a 441% average increase in tear production after LNS as a percent of baseline. After stimulation, right eyes had statistically significant greater increase in tear volumes than left eyes (p=0.028, Wilcoxon test). Post-stimulation right eye tear volumes were significantly greater compared to baseline (p=0.028, Wilcoxon test). Histopathologic examination of the lacrimal glands showed no discernible tissue damage from chronic neurostimulation. Additionally, there were no gross adverse effects on the general well-beings of the animals due to chronic stimulation.
Conclusions
Lacrimal nerve stimulation with an implantable pulse generator appears to increase aqueous tear production. Chronic LNS showed no histopathologic lacrimal gland damage. This study suggests LNS is a promising new treatment strategy to increase aqueous tear production.
The management of dry eye disease (DED) is a challenge in clinical practice. In United States, DED is a leading cause of eye discomfort and morbidity and accounts for approximately 30% of patients seeking eye care.1 Approximately 20 million Americans suffer from dry eyes2 and it is estimated that roughly 5 million patients, 50 years and older, have moderate to severe disease.3 This multi-factorial process is defined as a tear film disorder due to aqueous tear deficiency or excessive evaporation resulting in ocular discomfort, visual disturbance, and tear film instability.4 Tear film instability is frequently accompanied by increased tear osmolarity and subsequent inflammation of the ocular surface.4,5 In severe cases, corneal opacification and vascularization leads to a significant loss of vision.
The lacrimal functional unit controls the quality and quantity of aqueous tear secretion. Stern et al,6 presented this concept to describe the relationship between the ocular surface and lacrimal glands both in normal tear secretion and during inflammation.7 This unit comprises the lacrimal glands, ocular surface, and the interconnecting innervation. It is postulated that DED is an immune-mediated disorder and tear secretion is controlled by a neural reflex arc involving the central nervous system. In a healthy eye, stimulation of free nerve endings on the cornea and conjunctiva generate afferent nerve impulses through the ophthalmic branch of the trigeminal nerve (V1). These impulses travel to the midbrain where they synapse in the pons. The parasympathetic nervous system is largely responsible for the efferent branch of this loop. Efferent fibers from the superior salivary nucleus pass with the nervus intermedius, synapse in the pterygopalatine ganglion, travel through the inferior orbital fissure, and then join the lacrimal nerve to reach the main and accessory lacrimal glands.6–8
Disruption of this functional unit at any level can initiate a cascading sequence of inflammatory events, involving mitogen-activated protein (MAP) kinases, nuclear factor kappa beta (NFkB) signaling pathways, and the generation of inflammatory cytokines and matrix metalloproteinase (MMP), which will result in neural feedback inhibition.9 The interruption of neural input to the lacrimal gland leads to sensory isolation, T-cell activation, release of pro-inflammatory cytokines, and further lacrimal gland damage, neurogenic inflammation, and atrophy.10–12 The consequence of this self-perpetuating inflammatory cycle is a decrease in the quantity and quality of aqueous tear production.6,7
In the last two decades there has been a paradigm shift in dry eye management from lubricating the ocular surface with artificial tears, to strategies directed at inhibiting inflammatory factors that adversely influence the ability of glandular epithelia to produce tears.8,10 This has led to the development of topical cyclosporine (0.05% ophthalmic emulsion), the first FDA approved therapy for chronic dry eye.13 Cyclosporine reduces ocular surface inflammation by the inhibition of transcription factors required for cytokine production and T cell lymphocyte activation. While this treatment aims to diminish neural feedback inhibition by reducing ocular surface inflammation, it has shown only marginal efficacy. In a trial involving 1200 people, cyclosporine increased tear production in 15% of people; compared to 5% with placebo.14 Topical corticosteroids can also reduce ocular inflammation but have an unacceptable side effect profile for chronic use. The principal shortcoming of currently available pharmacologic treatments relates to the inherent limitation of these therapies to effectively bypass the neuronal inhibition of the lacrimal gland and directly stimulate the lacrimal nerve to increase aqueous secretion.
Neurostimulation is a promising therapeutic field of medicine that has proven effective in a wide variety of disorders including; epilepsy, chronic pain, depression, Parkinson’s disease, hearing loss, urinary urge incontinence, fecal incontinence, urinary retention and chronic headache.15–22 Neurostimulation involves modulation of the nervous system to restore, block, or augment nerve input to an effector organ or tissue. Implantable neurostimulators are pacemaker-sized devices that send electrical stimulation through an insulated electrode implanted near a central or peripheral nerve. They have been used in medicine with increasing frequency due to their safety, efficacy, and low risk profile. An implantable pulse generator (IPG) can be customized to the patient, reprogrammed telemetrically, and carries a low risk of infection. The device is silent, painless and biocompatible with human tissues.15–18 The intent of this study is to adopt this technology to override the ocular surface neural inhibition perpetuating the dry eye condition by direct lacrimal nerve stimulation (LNS) to increase tear secretion.
Methods
This investigation adheres to the guidelines endorsed by the Association for Research in Vision and Ophthalmology (ARVO) statement for the Use of Animals in Ophthalmic and Vision Research, and the investigators received prior approval from the Institutional Animal Care and Use Committee at the University of Miami. Six male Dutch Belted rabbits were used in the study. Each rabbit underwent surgical implantation of an implantable pulse generator (IPG) adjacent to the right lacrimal nerve. All surgical procedures were performed with the rabbits under anesthesia with aseptic technique. Antibiotic ointment was instilled over skin incisions after each procedure.
Quantification of Pre-stimulation Tear Volume (Baseline)
A real-time anterior segment ultra high-resolution spectral domain optical coherence tomography (UHR-OCT) was used to measure tear volume. The optical coherence tomography (OCT) light source was 840 nm with a bandwidth of 100 nm. The optical resolution was ~3 μm in the cornea. It was connected to a telecentric optical probe with a maximum 15-mm scanning width running at 24k A-scan per second. The telecentric design used light that was parallel at any scan spot for a wide scan and was specifically designed to capture the real spatial relationship of the tears over the ocular surface. The probe was mounted on a standard slit lamp with a digital video system.23–28 The UHR-OCT performed vertical 12 mm scans across the central cornea (apex), including the upper and/or lower tear menisci of both eyes, to quantify baseline tear volume.
Orbital Implantation of the Neurostimulator
The Advanced Bionics Precision™ Neurostimulator system is a 16-output multi-channel IPG with 55 mm (height), 46 mm (width), and 11 mm (thickness) dimensions and a hermetically sealed rechargeable battery power source.29 The IPG received radiofrequency (RF) programming signals from an external programming system and remote control. The IPG decoded the RF signals and delivered stimulation pulses to the lacrimal nerve via insulated output electrodes.
Dissection
After adequate anesthesia was administered with an intramuscular cocktail of ketamine 35 mg/kg (Vedco Inc., St. Joseph, MO. 64507), xylazine 5 mg/kg (Akorn, Inc., Decatur, IL 62522) and acepromazine 0.75 mg/kg (Vedco Inc., St. Joseph, MO. 64507), a small area of skin over the abdomen and right forehead was shaved. The abdomen was incised and a subcutaneous pocket was bluntly dissected large enough to house the IPG. Communication between the IPG and the external remote control was tested and confirmed. A straight stylet was tunneled in a subcutaneous plane to connect an insulated electrode from the abdominal IPG to a skin incision placed over the supraorbital border of the frontal bone. Blunt dissection was carried through the supraorbital foramina and the periorbita over the lacrimal gland. The insulated electrode was then positioned in contact with the posterior edge of the lacrimal gland, adjacent to the lacrimal nerve, along the orbital roof. The electrode was secured to the subcutaneous fascia with three 5-0-vicryl sutures and the skin incisions were closed. The left orbit served as the control.
Neurostimulation Protocol & Quantification of Post-stimulation Tear Volume
Twenty-four to forty-eight hours after implantation of the IPG into the right orbit, the baseline tear volumes were measured as previously described. The right lacrimal nerve was then stimulated using a remote control that sent RF signals to the IPG. An external programming system connected to the remote control was set to deliver stimulation with invariable pulse duration of 100 μs, electric current of 1.6 mAmp, a pulse frequency of 20 Hz, and a variable voltage of 5–8 volts for two minutes. Immediately following stimulation, UHR-OCT imaging of the tear meniscus was performed on the right eye. The right lacrimal nerve was again stimulated for two minutes at the same parameters and UHR-OCT imaging of the tear meniscus was performed on the left eye (control). This process was repeated at least three times for both eyes in all rabbits.
UHR-OCT Tear Volume Measurement
Image processing and data analysis were performed using custom software to yield tear meniscus variables.23–25,27,28 Six variables were obtained including upper tear meniscus curvature (UTMC), height (UTMH), and cross-sectional area (UTMA), and lower tear meniscus curvature (LTMC), height (LTMH), and cross-sectional area (LTMA). The upper tear meniscus volume (UTMV) was calculated by multiplying the upper lid length (mm) by the UTMA (mm2). The lower tear meniscus volume (LTMV) was calculated by multiplying the lower lid length (mm) by the LTMA (mm2). To use the two-dimensional image to estimate the area of the ocular surface that is curved in the third dimension, a multiplication factor of 1.294 was used for the UTMV and LTMV. Additionally, to account for the fact that the tear meniscus height is not uniform across the eyelid, both values were adjusted by a factor of 0.75.25 The final adjusted values of the UTMV and LTMV were then added together to yield the total tear meniscus volume (TTMV) of each eye.
Statistical Analysis
Data analysis was conducted with SPSS version 21 software (IBM Corporation, Armonk NY). Data were expressed as the mean (standard deviation); however, statistical comparisons were made with the nonparametric-paired Wilcoxon test due to the variability between animal responses. The criterion for statistical significance was P < 0.05.
Chronic Lacrimal Nerve Stimulation and Lacrimal Gland Histopathologic Analysis
Three rabbits underwent chronic stimulation of the right lacrimal nerve to determine if chronic stimulation would yield end organ damage to the lacrimal gland or surrounding tissues. Stimulation of the right lacrimal nerve occurred Monday through Friday, three times a day, with continuous electrical stimulation for a period of 20 minutes each session through four consecutive weeks. The external programming system was set to deliver stimulation to the lacrimal nerve with invariable pulse duration of 100 μS, electric current of 1.6 mAmp, pulse frequency of 10 Hz, and voltage of 2 volts. After four weeks of chronic stimulation, all three rabbits were euthanized with intravenous Euthanasia Solution 390 mg/ml, 1-ml/10 lbs. of body weight (Virbac Corporation, Fort Worth TX. 76161). The right and left orbits were harvested and sent for histopathologic evaluation by the Ophthalmic Pathology Laboratory of the Bascom Palmer Eye Institute. The tissue was fixed in 2.0% paraformaldehyde; 2.0% gluteraldehyde in 0.1-mmol/l cacodylate solution and embedded in paraffin. Eight-micron sections were stained with hematoxylin and eosin to evaluate the stroma and acini of the lacrimal gland and surrounding tissues after chronic stimulation.
Results
The total tear meniscus volumes (TTMV) for each rabbit’s right and left eyes before and after stimulation are illustrated in Table 1. Ultra high-resolution optical coherence tomography (UHR-OCT) of the right eyes showed a 441% average increase in tear production after lacrimal nerve stimulation (LNS) as a percentage of baselines. Pre-stimulation and post-stimulation UHR-OCT tear menisci images of the right eye of rabbits 1–3 are presented in Figure 1.
Table 1.
Pre-stimulation and post-stimulation Total Tear Meniscus Volumes (TTMV) for all rabbits. OD= Right eye, OS= Left eye
| Pre-stimulation TTMV (μl) | Post-stimulation TTMV (μl) | |
|---|---|---|
| Rabbit 1 TTMV OD | 1.203 | 4.328 |
| Rabbit 1 TTMV OS | 0.964 | 1.165 |
| Rabbit 2 TTMV OD | 3.668 | 16.013 |
| Rabbit 2 TTMV OS | 4.483 | 2.358 |
| Rabbit 3 TTMV OD | 2.649 | 30.328 |
| Rabbit 3 TTMV OS | 2.756 | 5.881 |
| Rabbit 4 TTMV OD | 1.600 | 11.260 |
| Rabbit 4 TTMV OS | 0.899 | 4.619 |
| Rabbit 5 TTMV OD | 1.087 | 7.061 |
| Rabbit 5 TTMV OS | 1.153 | 3.511 |
| Rabbit 6 TTMV OD | 2.125 | 5.447 |
| Rabbit 6 TTMV OS | 2.220 | 2.536 |
Figure 1.
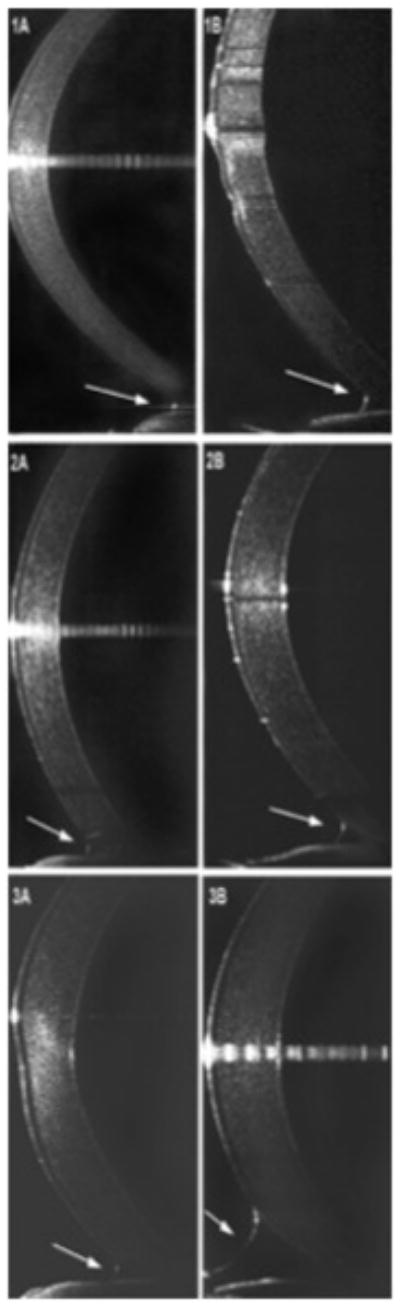
Ultra high-resolution optical coherence tomography (UHR-OCT) tear menisci images of rabbits 1–3. A. Pre-stimulation inferior tear meniscus (arrow), B. Post-stimulation inferior tear meniscus (arrow).
Pre-stimulation (Baseline) Tear Volumes of the Right (Experimental) and Left (Control) Eyes Compared
A scatter plot graph comparing the baseline tear volumes of the right and left eyes demonstrates that all six points fall close to the 1:1 line, indicating that there was little difference between the baseline measurements of the right and left eyes (Fig. 2). One of the experimental eyes measured 0.7 μl larger than its control and one of the control eyes measured 0.8 μl larger than its experimental eye. There was no statistical difference by the Wilcoxon non-parametric test (p=0.75).
Figure 2.
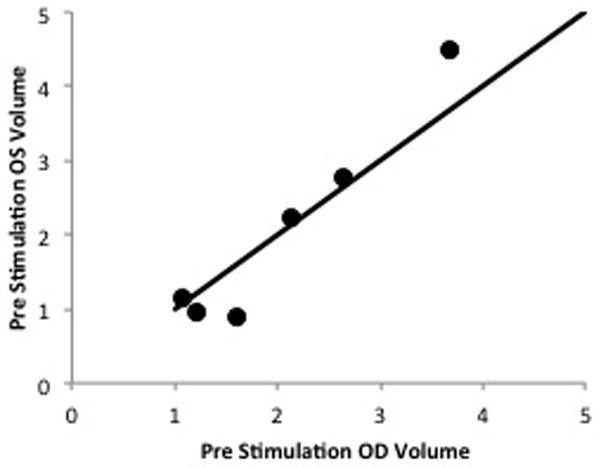
Scatter plot graph comparing baseline (pre-stimulation) tear volumes of the right and left eyes. No statistical difference was demonstrated by the Wilcoxon non-parametric test (p=0.75).
Pre-stimulation (Baseline) Tear Volumes Compared to Post-stimulation Tear Volumes (Right Eyes)
Baseline right eye tear volumes increased from an average of 2.06 (0.98) μl to 12.41 (9.78) μl after LNS. These findings were found to be statistically significant by the non-parametric Wilcoxon test (p=0.028). A scatter plot graph comparing the pre-stimulation and post-stimulation tear volumes of the right eyes shows that all points fall significantly above the 1:1 line, signifying that the post-stimulation tear volumes were notably higher than the pre-stimulation tear volumes (Fig. 3). There was no appreciable change in the tear volumes of the left eyes after LNS (p=0.12, Wilcoxon test).
Figure 3.
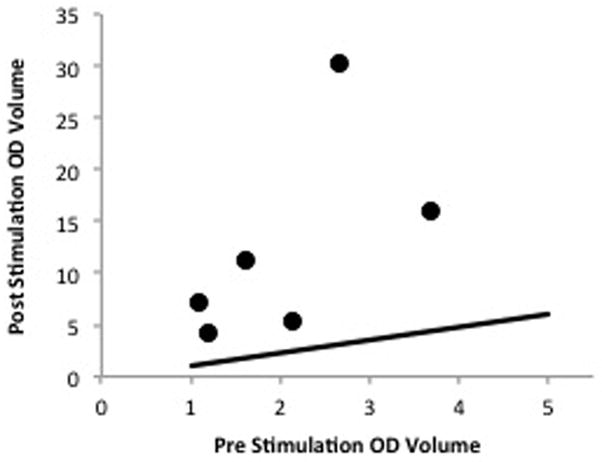
Scatter plot graph comparing pre-stimulation and post-stimulation tear volumes of the right eye. There was a statistically significant increase in tear volumes after stimulation, nonparametric Wilcoxon test (p=0.028).
Post-stimulation Tear Volume of Right Eyes (Experimental) Versus Left Eyes (Control)
There was a statistically significant increase in the post-stimulation tear volumes of right eye compared to the left eye in each rabbit. A scatter plot graph comparing the two eyes shows all points resided substantially above the 1:1 line, demonstrating that all six experimental eyes had increased tear volumes compared to the control eyes after right LNS (Fig. 4). The right eye minus left eye tear volume differences averaged −0.02 (0.50) μl prior to stimulation and increased to 9.06 (8.56) μl after stimulation (p=0.028, Wilcoxon test).
Figure 4.
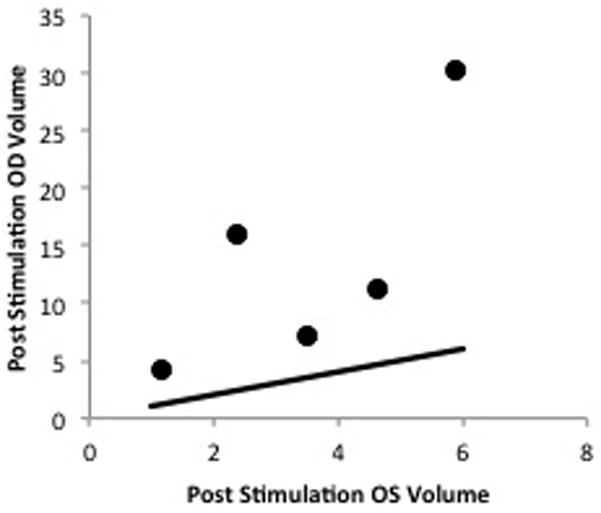
Scatter plot graph comparing post-stimulation tear volumes of the right and left eyes. There was a statistically significant increase in tear volumes of the right eyes compared to the left eyes after lacrimal nerve stimulation, nonparametric Wilcoxon test (p=0.028).
Chronic Lacrimal Nerve Stimulation and Histopathologic Analysis
There was no significant difference in body weight change among the three rabbits during chronic stimulation, suggesting that repetitive stimulation of the orbital tissues did not alter the feeding pattern or behavior of the animals. The overall growth was otherwise normal and there were no other demonstrable adverse effects to stimulation. Upon harvesting the lacrimal gland, the insulated electrodes were found to be within the right orbit adjacent to the lacrimal gland, indicating that little, if any, lead migration had occurred (Fig. 5). Histopathologic examination of the three rabbit’s right and left lacrimal glands revealed no discernible lymphocytic or inflammatory cell infiltration or atrophy to the stroma or acini of the lacrimal gland after repetitive stimulation (Fig. 6). Additionally, exenterated orbital tissues of the three rabbits showed normal orbital soft tissue structures without evidence of foreign body reaction, inflammation, hemorrhage, fungi, or bacteria.
Figure 5.

A. Insulated electrode adjacent to the right lacrimal nerve (arrow), B. Electrode along orbital roof after harvesting the lacrimal gland (arrow).
Figure 6.
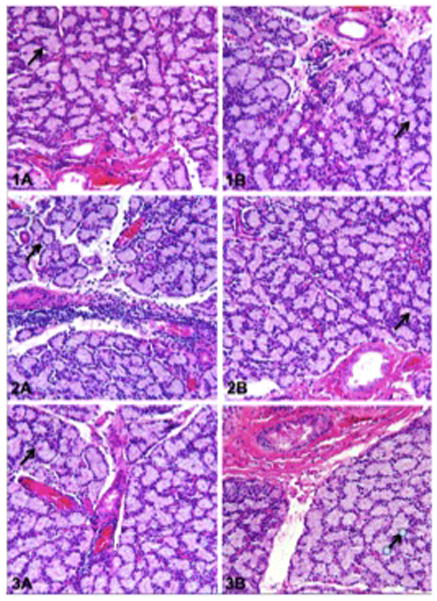
Histopathology of the lacrimal gland after chronic stimulation, Rabbits 1–3. Specimens exhibit no discernable inflammatory cell infiltration or atrophy of the lacrimal gland stroma or acini. A. Right (experimental) lacrimal gland with normal acinar units (arrow), B. Left (control) lacrimal gland with normal acinar units (arrow).
Discussion
This animal study introduces neurostimulation as a potential new treatment strategy for dry eye disease (DED) and demonstrates that direct lacrimal nerve stimulation (LNS) with an implantable pulse generator (IPG) can effect a 441% average increase in aqueous tear production. Our study design indicates that LNS is anatomically sound, safe, and technically feasible to perform with an IPG. The pathophysiologic mechanism is consistent with other neurostimulation strategies; similarly, our results suggest that chronic LNS produces no discernible deleterious histological evidence of end organ damage. This study also shows that ultra-high resolution optical coherence tomography (UHR-OCT) appears to be an effective non-contact measurement of tear volume quantification to assess the effect of lacrimal nerve stimulation.
Numerous animal models of dry eye have been developed to understand the pathophysiological mechanism underpinning both Sjogren and non-Sjogren’s keratoconjunctivitis sicca (KCS) and to explore new therapies for dry eye. In a review of published animal models, Barabino et al,30 concluded that currently there is no optimal model that can adequately reflect the complex pathophysiology of DED.30–31 This barrier is due to the various pathophysiologic mechanisms and the inflammatory cycle involved in DED. Previous studies to identify new treatments have been directed at interrupting the various points of this inflammatory cycle to precipitate disease control;13,14,32–34 however few studies have produced tear secretion with much success. Our animal study does not attempt to duplicate a dry eye animal model but is designed to bypass the inflammation induced neuronal reflex inhibition loop by targeting the parasympathetic motor innervation to the lacrimal gland. By directly stimulating the lacrimal nerve, the terminal link of the neuronal loop, the study design attempts to reverse the feedback inhibition input by circumventing the complex cascading sequence of events governing the tear production neural arc. No previous animal studies have attempted to target dry eye therapy through direct neurostimulation. Our data reveal that end neuron stimulation can produce an increase in tear secretion and lend support to this novel therapeutic approach.
The anatomical rationale to stimulate the lacrimal nerve for enhanced tear production is based on prior anatomical animal studies.35–40 These studies aimed to define the anatomy and functional innervation of the lacrimal gland by electrical nerve stimulation. Botelho et al,35 achieved stimulation by means of square electrical pulses, which were applied to the lacrimal nerve and the sphenopalatine ganglion of cats through chloride silver electrodes. They found that the lacrimal nerve as it approaches the lacrimal gland contains secretomotor fibers, which pass through the sphenopalatine ganglion, that when electrically stimulated produce a change in outflow from the lacrimal gland excretory duct. Arenson et al,40 determined that the minimum frequency necessary to induce lacrimal secretion in cats ranged from 2 to 4 Hz and maximal secretion occurred between 7–15 Hz. Our animals were stimulated with and without anesthesia to select parameters that maximized tear volume secretion with no apparent evidence of pain or muscle contraction in the rabbit while awake. When the voltage of stimulation was above 10 Hz flinching was observed while awake; therefore the voltage was kept between 5–8 Hz.
The pathophysiological rationale for LNS to increase tear secretion is based on previous animal and clinical studies using sacral and pudendal nerve stimulation to increase bladder contraction. Yoo et al,41 demonstrated that pudendal nerve stimulation, in spinal cord transected cats, evoked sustained bladder contraction dependent on stimulation frequency. Further clinical studies confirmed this finding in patients with urinary retention. In a five-year prospective, multicenter trial, Van Kerrebroeck et al,42 found that sacral nerve stimulation to produce bladder contraction is a safe, effective, and minimally invasive method that provides long-term relief for appropriately selected patients with refractory urinary retention. Five years after implantation, 71% of patients with urinary retention had persistent successful outcomes. These long-term results suggest that chronic stimulation of a terminal secretomotor nerve does not lead to end organ damage or atrophy. Our four-week chronic LNS trial evinced a similar finding. This was confirmed by histopathologic analysis of the lacrimal gland after repetitive stimulation that showed no lymphocytic or inflammatory cell infiltration or atrophy of the stroma or acini.
Difficulties in assessing the effectiveness of dry eye treatments lie in the absence of a universally accepted method of measuring the quality and quantity of tear production. A variety of diagnostic tests are in common clinical usage, but there is no consensus on which combination of tests should be used to evaluate tear volume in dry eyes. More commonly practiced methods of quantifying tear volume include Schirmer tear tests, Rose Bengal tests, slit-lamp measured lower tear meniscus height and tear break up time measurements; however these tests have shown wide variability, low reliability, and poor correlation with patients’ subjective symptoms.43–46 Due to the lack of standardization and variability of these techniques to accurately measure tear volume, we selected a new method of tear volume quantification, UHR-OCT. The current literature on tomographic characterization of the tear film and tear menisci indicates that parameters produced by OCT are good quantitative indicators of tear volume.23–28,43,47–56 The UHR-OCT imaging modality used in this study is a highly reliable tool for imaging the ocular surface tear film and calculating the tear volume.25–28,49 The non-contact imaging feature coupled with its reported accuracy and reproducibility makes this tool suitable for objective tear volume measurements in our animal design.
The putative mechanism of DED is a complex immune mediated inflammatory condition in which cytokines disrupt the normal neural control of tearing by interrupting the secretomotor nerve impulses to the lacrimal gland. The benefit of LNS is the ability to override the feedback loop inhibition of the neural arc. While the neurostimulation strategy in this study demonstrates an increase in tear production and no apparent structural damage to the lacrimal gland, it is unclear whether repetitive secretomotor impulses to the lacrimal gland will lead to suppression of the ocular surface inflammation that initiates the immune cascade, thereby restoring the normal neuronal control. Conversely, it is unclear whether neurostimulation will lead to a deleterious increase in neurogenic inflammation manifesting in a surge of T-cell activation and cytokine release into the tears.
Limitations to this study include the small number of animals examined. However, because the increase in tear volume was marked and consistent, the power of the study was sufficient to achieve statistical significance. Additionally, our study only assessed the quantity of tears produced, not the quality of tears. Qualitative analysis of the stimulation-secreted tears is necessary to provide a better understanding of the complex interplay between neuronal stimulation and tear physiology. Other limitations include the study duration of four weeks, perhaps this short duration is insufficient to assess the effect of repetitive stimulation to the lacrimal nerve, gland, and surrounding soft tissues. We were also limited by the orbital anatomy of the rabbit, which precludes isolated LNS without simultaneously stimulating the body of the lacrimal gland. Due to the variable lacrimal nerve anatomy in the rabbit, we elected to place the lead wire adjacent to the posterior lacrimal gland in the superior orbit with an electromagnetic field large enough to stimulate the lacrimal nerve at its terminal innervation to the lacrimal gland. With this technique, suboptimal lead placement may have resulted in unequal distances between the lacrimal nerve and lead wire and could account for the high variability of tear production between the rabbits. Finally, the IPG system used is bulky, requiring considerable surgical manipulation to expose the lacrimal nerve and is not ideal for translation into clinical practice. A miniature implantable unit with external wireless control would be more desirable.
The future direction is to employ an implantable electrical stimulation system called a microstimulator that contains a wireless, rechargeable pulse generator and electrode together in a miniature size, ideal for implantation into the lacrimal gland fossa.16–19 The microstimulator will be designed for implantation in the orbit using a minimally invasive approach for direct LNS. We will also utilize a tear osmolarity measurement system in conjunction with UHR-OCT tear volume measurements to evaluate the physiologic homeostasis of tear composition and accurately assess tear volume. We will incorporate other tear volume measurement methods, including Schirmer tests and TBUT, to correlate and validate our measurements with the UHR-OCT system. Finally, we need to perform long-term stimulation to better evaluate the effect of repetitive stimulation on the lacrimal functional unit.
In conclusion, we present a proof-of-concept animal study using an IPG to stimulate the lacrimal nerve to induce an increase in aqueous tear volume production. Further studies with a microstimulator, enhanced metrics, tear osmolarity measurement, and inflammatory biomarker assay are needed to assess this new treatment alternative.
Acknowledgments
Financial support: This work was supported in part by: NIH Center Core Grant P30EY014801; Research to Prevent Blindness Unrestricted Grant, Inc., New York, New York; Department of Defense (DOD - Grant W81XWH-09-1-0675); Plum Foundation, Los Angeles, California; Boston Scientific Neuromodulation Corporation, Valencia, California, and the Dr. Nasser Ibrahim Al-Rashid Orbital Vision Research Fund. The sponsor or funding organizations had no role in the design or conduct of this research.
Footnotes
Meeting presentation: American Society of Ophthalmic Plastic & Reconstructive Surgery Fall Meeting. New Orleans, November 2014.
Proprietary interest statement: No conflicting relationship exists for any author.
References
- 1.American Academy of Ophthalmology. Dry Eye syndrome-Preferred practice patterns. American Academy of Ophthalmology; 2003. [Google Scholar]
- 2.Market ScopeReport on the global dry eye market. St. Louis, MO: 2004. [Google Scholar]
- 3.Schaumberg DA, Sullivan DA, Buring JE, Dana MR. Prevalence of dry eye syndrome among US women. Am J Ophthalmol. 2003;136:318–326. doi: 10.1016/s0002-9394(03)00218-6. [DOI] [PubMed] [Google Scholar]
- 4.Lemp MA. Report of the National Eye Institute/Industry Workshop on Clinical Trials in Dry Eyes. CLAO J. 1995;21:221–232. [PubMed] [Google Scholar]
- 5.Lemp MA, Baudouin C, Baum J, et al. The definition and classification of dry eye disease: report of the Definition and Classification Subcommittee of the International Dry Eye Work Shop (2007) Ocular Surf. 2007;5:75–92. doi: 10.1016/s1542-0124(12)70081-2. [DOI] [PubMed] [Google Scholar]
- 6.Stern ME, Beuerman RW, Fox RI, Gao J, Mircheff AK, Pflugfelder SC. The Pathology of Dry Eye: The Interaction Between the Ocular Surface and Lacrimal Glands. Cornea. 1998;17:584–589. doi: 10.1097/00003226-199811000-00002. [DOI] [PubMed] [Google Scholar]
- 7.Stern ME, Gao J, Siemasko KF, et al. The role of the lacrimal functional unit in the Pathophysiology of dry eye. Exp Eye Res. 2004;78:409–416. doi: 10.1016/j.exer.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 8.Report of the Epidemiology Subcommittee of the International Dry Eye Work Shop: The epidemiology of dry eye disease. Ocul Surf. 2007;5:93–107. doi: 10.1016/s1542-0124(12)70082-4. [DOI] [PubMed] [Google Scholar]
- 9.De Paiva CS, Corrales RM, Villarreal AL, et al. Corticosteroid and doxycycline suppress MMP-9 and inflammatory cytokine expression, MAP K activation in the corneal epithelium in experimental dry eye. Exp Eye Res. 2006;83:526–535. doi: 10.1016/j.exer.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 10.Management and Therapy Subcommittee. Management and therapy of dry eye disease: report of the Management and Therapy Subcommittee of the International Dry Eye Work-Shop (2007) Ocul Surf. 2007;5:163–178. doi: 10.1016/s1542-0124(12)70085-x. [DOI] [PubMed] [Google Scholar]
- 11.Luo L, Li DQ, Doshi A, et al. Experimental dry eye stimulates production of inflammatory cytokines and MMP-9 and activates MAP K signaling pathways on the ocular surface. Invest Ophthalmol Vis Sci. 2004;45:4293–4301. doi: 10.1167/iovs.03-1145. [DOI] [PubMed] [Google Scholar]
- 12.Stern ME, Pflugfelder SC. Inflammation in dry eye. Ocular Surf. 2004;2:124–130. doi: 10.1016/s1542-0124(12)70148-9. [DOI] [PubMed] [Google Scholar]
- 13.Roberts CW, Carniglia PE, Brazzo BG. Comparison of topical cyclosporine, punctal occlusion, and a combination for the treatment of dry eye. Cornea. 2007;26:805–809. doi: 10.1097/ICO.0b013e318074e460. [DOI] [PubMed] [Google Scholar]
- 14.Meadows M. Dealing with Dry Eye. FDA Consumer Magazine; 2005. [PubMed] [Google Scholar]
- 15.Dupont AC, Bagg SD, Baker L, Creasy JL, Romano C, Romano D, Richmond FJR, Loeb GE. First patients with BION implants for therapeutic electrical stimulation. Neuromodulation. 2004;7:38–47. doi: 10.1111/j.1525-1403.2004.04005.x. [DOI] [PubMed] [Google Scholar]
- 16.Loeb GE, Richmond FJR, Singh J, Peck RA, Tan W, Zou Q, Sachs N. RF-Powered BIONs for Stimulation and Sensing, Proc. 26th Ann. Intl. Conference IEEE Engineering in Medicine and Biology Society; San Francisco. Sept. 1–5; 2004. [DOI] [PubMed] [Google Scholar]
- 17.Baker L, Waters R, Winstein C, Kaplan H, Tran W, Richmond FJR, Loeb GE. Clinical Applications of BION(TM) Microstimulators. First International Conf. on Neural Interface and Control; Wuhan, China. May 26–28, 2005; 2005. [Google Scholar]
- 18.Dupont AC, Bagg SD, Chun S, Creasy JL, Romano C, Romano D, Waters RL, Wederich CL, Richmond FJR, Loeb GE. IFESS. LJUBLJANA; SLOVENIA: 2002. Clinical Trials of BION™ Microstimulators. [Google Scholar]
- 19.Schwedt TJ, Dodick DW, Hentz J, et al. Occipital nerve stimulation for chronic headache: long-term safety and efficacy. Cephalalgia. 2007;27:153–157. doi: 10.1111/j.1468-2982.2007.01272.x. [DOI] [PubMed] [Google Scholar]
- 20.Saper JR, Dodick DW, Silberstein SD, et al. Occipital nerve stimulation for the treatment of intractable chronic migraine headache: ONSTIM feasibility study. Cephalalgia. 2011;31:271–285. doi: 10.1177/0333102410381142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tubaro A. Sacral root neuromodulation in the treatment of refractory urinary urge incontinence: a prospective randomized clinical trial. Current Opinion in Urology. 2000;10:489–490. doi: 10.1159/000020134. [DOI] [PubMed] [Google Scholar]
- 22.Tjandra JJ, Lim JF, Matzel K. Sacral nerve stimulation: An emerging treatment for fecal incontinence. ANZ Journal of Surgery. 2004;74:1098–1106. doi: 10.1111/j.1445-1433.2004.03259.x. [DOI] [PubMed] [Google Scholar]
- 23.Wang J, Aquavella JV, Palakuru JR, Chung S, Feng C. Relationships between central tear film thickness and tear menisci of the upper and lower eyelids. Investigative Ophthalmology & Visual Science. 2006;47:4349–4355. doi: 10.1167/iovs.05-1654. [DOI] [PubMed] [Google Scholar]
- 24.Palakuru JR, Wang J, Aquavella JV. Effect of blinking on tear dynamics. Investigative Ophthalmology & Visual Science. 2007;48:3032–3037. doi: 10.1167/iovs.06-1507. [DOI] [PubMed] [Google Scholar]
- 25.Wang J, Aquavella JV, Palakuru JR, Chung S. Repeated measurements of dynamic tear distribution on the ocular surface after instillation of artificial tears. Investigative Ophthalmology & Visual Science. 2006;47:3325–3329. doi: 10.1167/iovs.06-0055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shen M, Li J, Wang J, et al. Upper and lower tear menisci in the diagnosis of dry eye. Invest Ophthalmol Vis Sci. 2009;50:2722–2726. doi: 10.1167/iovs.08-2704. [DOI] [PubMed] [Google Scholar]
- 27.Wang J, Abou Shousha M, Perez VL, Karp CL, Yoo SH, Shen M, Cui L, Humeric V, Du C, Zhu D, Chen Q, Li M. Ultra-high resolution optical coherence tomography for imaging the anterior segment of the eye. Ophthalmic Surg Lasers Imaging. 2011;42:S15–27. doi: 10.3928/15428877-20110627-02. [DOI] [PubMed] [Google Scholar]
- 28.Li M, Du C, Zhu D, Shen M, Cui L, Wang J. Daytime variations of tear osmolarity and tear meniscus volume. Eye Contact Lens. 2012;38:282–287. doi: 10.1097/ICL.0b013e31825fed57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.http://www.fda.gov/MedicalDevices/ProductsandMedicalProcedures/DeviceApprovalsandClearances/Recently-ApprovedDevices/ucm081164.htm
- 30.Barabino S, Dana MR. Animal models of dry eye: a critical assessment of opportunities and limitations. Invest Ophthalmol Vis Sci. 2004;45:1641–1646. doi: 10.1167/iovs.03-1055. [DOI] [PubMed] [Google Scholar]
- 31.Barabino S, Chen W, Dana MR. Tear film and ocular surface tests in animal models of dry eye: uses and limitations. Experimental Eye Research. 2004;79:613–621. doi: 10.1016/j.exer.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 32.Scifo C, Barabino S, De Pasquale G, Blanco AR, Mazzone MG, Rolando M. Effects of a New Lipid Tear Substitute in a Mouse Model of Dry Eye. Cornea. 2010;29:802–806. doi: 10.1097/ICO.0b013e3181ca327e. [DOI] [PubMed] [Google Scholar]
- 33.Nakamura S, Okada S, Umeda Y, Saito F. Development of a Rabbit Model of Tear Film Instability and Evaluation of Viscocity of Artificial Tear Preparations. Cornea. 2004;23:390–397. doi: 10.1097/00003226-200405000-00015. [DOI] [PubMed] [Google Scholar]
- 34.Gao J, Schwalb TA, Adeeo JV, Ghosn CR, Stern ME. The Role of Apoptosis in the Pathogenesis of Canine Keratoconjunctivitis Sicca: The Effect of Topical Cyclosporin A Therapy. Cornea. 1998;17:654. doi: 10.1097/00003226-199811000-00014. [DOI] [PubMed] [Google Scholar]
- 35.Botelho S, Mituhiko H, Fuenmayor N. Functional Innervation of the Lacrimal Gland in the Cat. Origin of the Secretomotor Fibers in the Lacrimal Nerve. Arch Ophthal Vol. 1966;76:581–589. doi: 10.1001/archopht.1966.03850010583019. [DOI] [PubMed] [Google Scholar]
- 36.Lundberg A. The Electrophysiology of the Submaxillary Gland of the Cat. Acta Physiol Scand. 1955;35:1–25. doi: 10.1111/j.1748-1716.1955.tb01258.x. [DOI] [PubMed] [Google Scholar]
- 37.Lundberg A. Secretory Potentials in the Sublingual Gland of the Cat. Acta Physiol Scand. 1957;40:21–34. doi: 10.1111/j.1748-1716.1957.tb01475.x. [DOI] [PubMed] [Google Scholar]
- 38.Arenson MS, Wilson H. The parasympathetic secretory nerves of the lacrimal gland of the cat. Journal of Physiology. 1971;217:201–212. doi: 10.1113/jphysiol.1971.sp009566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wikberg JE. Synthesis of 3H-acetylcholine in the rabbit lacrimal gland and its release by electrical field stimulation. Acta Physiologica Scandinavica. 1979;105:108–113. doi: 10.1111/j.1748-1716.1979.tb06318.x. [DOI] [PubMed] [Google Scholar]
- 40.Arenson MS, Wilson H. The peripheral parasympathetic innervation of the cat lacrimal gland. British Journal of Pharmacology. 1970;39:242–243. [PMC free article] [PubMed] [Google Scholar]
- 41.Yoo PB, Woock JP, Grill WM. Bladder activation by selective stimulation of pudendal nerve afferents in the cat. Experimental Neurology. 2008;212:218–255. doi: 10.1016/j.expneurol.2008.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Van Kerrebroek P, Van Voskusilen A, Heesakkers J, et al. Results of sacral neuromodulation therapy for urinary voiding dysfunction: outcomes of prospective, worldwide clinical study. J Urol. 2007;178:2029–2034. doi: 10.1016/j.juro.2007.07.032. [DOI] [PubMed] [Google Scholar]
- 43.Nguyen P, Hugang D, Li Y, Sadda SR, Ramos S, Pappuru RR, Yiu SC. Correlation Between Optical Coherence Tomography-Derived Assessments of Lower Tear Meniscus Parameters and Clinical Features of Dry Eye Disease. Cornea. 2012;31:680–685. doi: 10.1097/ICO.0b013e3182261577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nichols KK, Mitchell GL, Zadnik K. The repeatability of clinical measurements of dry eye. Cornea. 2004;23:272–285. doi: 10.1097/00003226-200404000-00010. [DOI] [PubMed] [Google Scholar]
- 45.Nichols KK, Nichols JJ, Mitchell GL. The lack of association between signs and symptoms in patients with dry eye disease. Cornea. 2004;23:762–770. doi: 10.1097/01.ico.0000133997.07144.9e. [DOI] [PubMed] [Google Scholar]
- 46.Schein OD, Tielsch JM, Munõz B, et al. Relation between signs and symptoms of dry eye in the elderly. A population-based perspective. Ophthalmology. 1997;104:1395–1401. doi: 10.1016/s0161-6420(97)30125-0. [DOI] [PubMed] [Google Scholar]
- 47.Park D, Lew H, Lee SY. Tear meniscus measurement in nasolacrimal duct obstruction patient with Fourier-domain optical coherence tomography: novel three-point capture method. Acta Ophthalmologica. 2012;90:783–787. doi: 10.1111/j.1755-3768.2011.02183.x. [DOI] [PubMed] [Google Scholar]
- 48.Qiu X, Gong L, Lu Y, Jin H, Robitaille M. The diagnostic significance of Fourier-domain optical coherence tomography in Sjogren syndrome, aqueous tear deficiency and lipid tear deficiency patients. Acta Ophthalmologica. 2012;90:e359–366. doi: 10.1111/j.1755-3768.2012.02413.x. [DOI] [PubMed] [Google Scholar]
- 49.Cui L, Wang J, Perez VL, Shen M, Yuan Y, Wang M. Visualization of the Precorneal Tear Film Using Ultrahigh Resolution Optical Coherence Tomography in Dry Eye. Eye & Contact Lens: Science & Clinical Practice. 2012;38:240–244. doi: 10.1097/ICL.0b013e318257a108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Huang D, Swanson EA, Lin CP, et al. Optical coherence tomography. Science. 1991;254:1178–1181. doi: 10.1126/science.1957169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Huang D, Izatt JA, Yasuno Y, et al. Future direction of anterior segment optical coherence tomography. In: Steinert RF, Huang D, editors. Anterior Segment Optical Coherence Tomography Thorofare. NJ: SLACK Inc; 2008. pp. 165–173. [Google Scholar]
- 52.Zhou S, Li Y, Lu AT, et al. Reproducibility of tear meniscus measurement by Fourier-domain optical coherence tomography: a pilot study. Ophthalmic Surg Lasers Imaging. 2009;40:442–447. doi: 10.3928/15428877-20090901-01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bitton E, Keech A, Simpson T, et al. Variability of the analysis of the tear meniscus height by optical coherence tomography. Optom Vis Sci. 2007;84:903–908. doi: 10.1097/OPX.0b013e3181560ba8. [DOI] [PubMed] [Google Scholar]
- 54.Ibrahim OM, Dogru M, Takano Y, et al. Application of visante optical coherence tomography tear meniscus height measurement in the diagnosis of dry eye disease. Ophthalmology. 2010;117:1923–1929. doi: 10.1016/j.ophtha.2010.01.057. [DOI] [PubMed] [Google Scholar]
- 55.Johnson ME, Murphy PJ. The agreement and repeatability of tear meniscus height measurement methods. Optom Vis Sci. 2005;82:1030–1037. doi: 10.1097/01.opx.0000192352.78935.e0. [DOI] [PubMed] [Google Scholar]
- 56.Savini G, Barboni P, Zanini M. Tear meniscus evaluation by optical coherence tomography. Ophthalmic Surg Lasers Imaging. 2006;37:112–118. [PubMed] [Google Scholar]


