Abstract
Purpose
To investigate the safety, tolerability and efficacy of subconjunctival sirolimus injections as a treatment for active, autoimmune, non-necrotizing, anterior scleritis.
Design
Phase I/II, single-center, open-label, non-randomized, prospective pilot study.
Methods
Five participants with active, autoimmune, non-necrotizing anterior scleritis with scleral inflammatory grade of ≥1+ in at least one quadrant with a history of flares were enrolled. A baseline injection was given, with the primary outcome measure of at least a 2-step reduction or reduction to grade zero in the study eye by eight weeks. Secondary outcomes included changes in visual acuity and intraocular pressure, ability to taper concomitant immunosuppressive regimen, and number of participants who experienced a disease flare requiring re-injection. Safety outcomes included the number and severity of systemic and ocular toxicities, and vision loss ≥ 15 ETDRS letters. The study included six visits over four months with an extension phase to one year for participants who met the primary outcome.
Results
All participants [N=5, 100% (95% CI [0.60, 1.00])] met the primary outcome in the study eye by the Week 8 visit. There was no significant change in mean visual acuity or intraocular pressure. Three out of five patients (60%) experienced flares requiring re-injection. No systemic toxicities were observed. Two participants (40%) experienced a localized sterile inflammatory reaction at the site of the injection which resolved without complication.
Conclusions
Subconjunctival sirolimus leads to a short-term reduction in scleral inflammation, though relapses requiring re-injection do occur. There were no serious adverse events, though a local sterile conjunctival inflammatory reaction was observed.
Scleritis is a chronic, painful, and destructive inflammatory disorder that can be associated with systemic disease, and less commonly, an infectious etiology. Scleritis can lead to ocular complications such as keratitis, uveitis, glaucoma and exudative retinal detachment.1,2 A classification scheme of scleritis was first proposed by Watson and Hayreh and is based on anatomy and appearance. They classified it as anterior or posterior and further subdivided into diffuse, nodular, and necrotizing scleritis with or without inflammation (scleromalacia perforans).3 Anterior scleritis is the most common form (80-85%), with diffuse and nodular forms occurring almost equally. It is often associated with severe pain and may be associated with sight-threatening complications.4
Non-infectious or autoimmune scleritis is thought to arise from immune-mediated mechanisms that are not well understood. Histopathologic studies from patients with necrotizing and recurrent non-necrotizing scleritis indicate the presence of vasculitis with fibrinoid necrosis and neutrophil invasion, as well as an increase in inflammatory cells, mainly activated T-cells and macrophages.5,6
Current treatment for scleritis is based on a stepwise approach beginning with topical corticosteroids and oral non-steroidal anti-inflammatory drugs (NSAIDs) for mild scleritis followed by systemic corticosteroids and/or immunosuppressive treatment for more severe disease.7-10 Periocular steroid injections have been used in several studies for non-necrotizing anterior scleritis. Their use has been controversial due to concern for scleral melting; though in recent studies there are no reported cases of scleral thinning or necrosis.11-13
Sirolimus, an mTOR (mammalian target of rapamycin) inhibitor, exerts its effect by a mechanism that is distinct from other immunosuppressive agents.14 It suppresses cytokine-driven T-cell proliferation and inhibits the production, signaling, and activity of many growth factors relevant to scleritis. Sirolimus tablets and oral solution are currently approved by the Food and Drug Administration (FDA) for the prevention of transplant rejection.15 Subconjunctival sirolimus offers the advantage of local delivery of medication for potential control of acute scleral inflammation without the concern of scleral thinning, cataract, or glaucoma, unlike periocular corticosteroid injections.
Methods
This was a phase I/II non-randomized, prospective single-center study that evaluated subconjunctival sirolimus as a treatment for active, anterior, autoimmune, non-necrotizing anterior scleritis. The study protocol was reviewed and approved by the Institutional Review Board of the National Institutes of Health, a HIPAA-compliant institution, and all procedures conformed to the tenets of the Declaration of Helsinki (Clinical Trials registration:NCT01517074; NEI protocol ID: 12-EI-0057). Informed consent was obtained from all participants at the time of enrollment. All participants received subconjunctival sirolimus in the study eye at baseline. Four participants received a single 15-μL (660-μg) subconjunctival injection and one participant received two 15-μL (660-μg) injections in the affected quadrants. Those who still demonstrated active inflammation, incomplete or no response to initial injection, or experienced a flare-up after the initial injection were eligible for repeat injections in the study eye at or after Week 4 if there was a flare-up. A flare-up was defined by a ≥1-step increase in scleral inflammation.
If greater than two quadrants were involved, two 15-μL (660-μg) injections were given in two quadrants 180° apart (total dose of 30-μL or 1,320-μg). The study duration was four months, with the option of extension to one year for pro re nata dosing for those who had a favorable response and met the primary outcome by 8 weeks. The study included six visits for the first four months (Baseline, Weeks 2, 4, 8, 12, and 16) and additional visits every eight weeks for the extension portion of the study (Weeks 24, 32, 40, and 48) followed by a final visit at Week 52. The last possible injection was allowed at 40 weeks to allow safety visits at 48 and 52 weeks.
Inclusion and Exclusion Criteria
Inclusion criteria included age ≥ 18 years and a diagnosis of active, anterior, autoimmune, non-necrotizing anterior scleritis. The study eye was required to have ≥1+ scleritis in at least one quadrant based on the NEI Scleritis Grading Scale.16 If taking immunosuppressive medications, the participants must have been on a stable regimen without increase, decrease and/or start of new medications for at least four weeks prior to enrollment. Participants were also required to have tried therapy such as oral NSAIDs, oral or topical corticosteroids or immunosuppressive medications in the past. Participants were required to have visual acuity (VA) of 20/640 or better in the study eye.
Important exclusion criteria included active intraocular infection in either eye, history of cancer (other than a non-melanoma skin cancer) diagnosed within the past five years, or active systemic inflammation requiring immediate addition of or increase in systemic anti-inflammatory medications. Participants who were pregnant or lactating, or those who refused to use contraception during the study, were also excluded.
Ophthalmic and Medical Evaluations
At all visits, participants underwent a complete ocular examination that included visual acuity assessment using the standardized Early Treatment Diabetic Retinopathy Study (ETDRS) refraction protocol at four meters, vital signs, concomitant medications assessment, adverse event assessment, intraocular pressure, slit lamp examination, dilated fundus examination, standardized scleral photographs, complete blood count with differential, basic metabolic panel, and urinalysis.
Primary, secondary and safety outcomes
The primary efficacy outcome was a 2-step reduction in scleritis grading out of a scale of 0 to 4+ (where 0.5+ is recognized as an ordinal step between 1+ and 0+) or reduction to grade 0 within 8 weeks, according to the standardized photographic grading system developed at NEI. Secondary outcomes included mean and median change in visual acuity via ETDRS, step changes in scleral inflammation according to the standardized photographic grading system at all follow-up visits, number of participants who experienced a disease flare as defined by a 1-step increase in scleral inflammation at all follow-up visits, mean and median number of participants needing at least one additional injection, and the number of days between the first injection to the second injection, and finally, the number of participants who were successfully tapered off one of more systemic immunosuppressive medications or tapered the dose of prednisone (≤10mg) after Week 16.
Safety outcomes were made routinely during the study, with a review of the previous visit interval performed at each scheduled visit. Safety outcomes included the number and severity of systemic and ocular toxicities and adverse events, proportion of participants with loss of ≥ 15 ETDRS letters at any follow-up visit and the number of participants who experienced a substantial rise in elevated intraocular pressure at any follow-up visit.
Study Drug Administration
All participants received a subconjunctival sirolimus (15-μL, 600-μg) injection on the day of study enrollment. One participant received two injections (each 15-μL, 600-μg) due to multiple non-adjacent quadrant involvement. Subconjunctival sirolimus injections were prepared for injection according to the manufacturer’s guidelines. Briefly, the study drug was thawed immediately prior to use, drawn into the syringe using sterile technique (0.3cc insulin Becton Dickinson [BD] syringe), and following topical anesthesia, the needle was engaged approximately 5 mm away from the intended location. All participants received 0.3% ofloxacin drops immediately following the procedure and three times daily for two days. Topical treatments and systemic immunosuppressives, including corticosteroids, were maintained at the baseline dose or reduced, not increased, for the study duration.
Results
Five participants with active, anterior, autoimmune, non-necrotizing anterior scleritis were enrolled in this phase I/II trial. There were 3 female and 2 male participants; the average age was 50.6 years (range: 25-65). The severity of inflammation ranged from grade 1+ to grade 3+ based on the NEI Scleritis Grading Scale, and 4 out of 5 participants had multiple quadrants involved at baseline. Four out of 5 participants were on stable doses of immunosuppressive medications, and one was on no other therapy.
All participants (N=5, 100%) met the primary efficacy outcome and achieved at least a 2-step reduction or reduction to grade zero inflammation in the study eye by the Week 8 visit. This was achieved with only one injection given at baseline. Mean visual acuity at baseline was 84.6 ETDRS letters and it was 84.4 at the end of the study. No participant experienced more than two line changes. Three out of five participants (60%) experienced disease flares requiring re-injection, and mean number of days until the first re-injection was 65.3 days (range: 28-84).
No systemic toxicities were observed, and none of the patients experienced a significant rise (≥ 10mmHg) in intraocular pressure. One out of four participants who was on concomitant immunosuppressives was able to successfully taper off therapy during the study.
Throughout the study there were no serious adverse events attributable to the study drug. However, two participants (40%) experienced a localized sterile inflammatory reaction at the site of the subconjunctival injection which resolved without complication. These are described in further detail below. One of these participants withdrew from the study during the extension phase despite resolution.
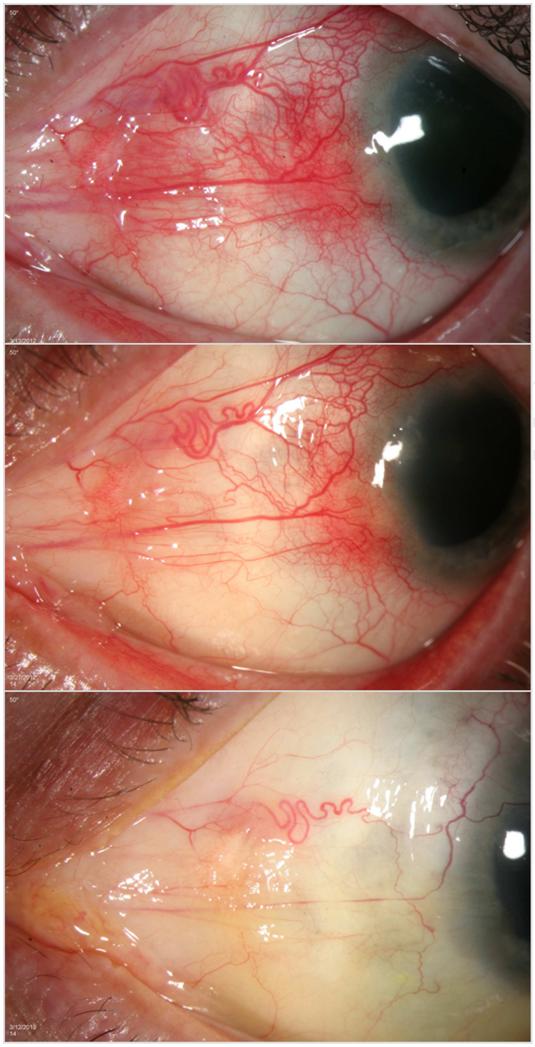
Participant 1 is a 63 year old Caucasian male without known systemic autoimmune disease who presented with grade 3+ inflammation in the superonasal and inferonasal quadrants at the baseline visit. He was on stable doses of methotrexate and mycophenolate mofetil at the time of enrollment. After one injection, his inflammation improved to grade 1+ and 0.5+ in the same quadrants, respectively, after two weeks. At four weeks, his inflammation returned to grade 2+ and 2+, meeting criteria for re-injection. He was re-injected monthly for five months for a total of five injections and by week 32, he achieved quiescence with grade 0 inflammation in all quadrants, which he maintained throughout the duration of the study (one year) (Figure 1).
Figure 1.
Response to treatment with subconjunctival sirolimus for the treatment of Autoimmune Non-Necrotizing Anterior Scleritis, Participant 1
The top panel, middle panel, and bottom panel show Participant 1 at Baseline, Week 2, and Week 52, respectively.
Participant 2 is a 25 year old Caucasian male without known systemic autoimmune disease who presented with grade 1+ inflammation in the superotemporal and inferotemporal quadrants at the baseline visit. He was not on systemic immunosuppressive medications and pain was not a major complaint for him. After one injection, he achieved quiescence with grade 0 inflammation in all quadrants, which was maintained throughout the duration of the study (one year).
Participant 3 is a 65 year old African American female with a history of rheumatoid arthritis who presented with grade 3+ inflammation in the superotemporal and inferotemporal quadrants. She was on a stable dose of adalimumab at the time of enrollment. After one injection, her inflammation improved to grade 1+ in the same quadrants, after four weeks. She remained with this level of inflammation until week 40, when she achieved complete quiescence without further treatment, with grade 0 inflammation in all quadrants and remained without inflammation throughout the duration of the study (one year).
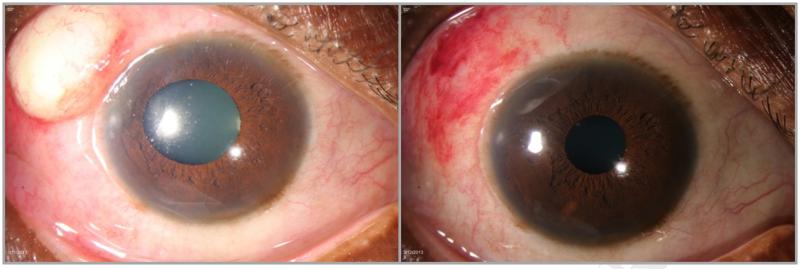
Participant 4 is a 50 year old African American female with a history of rheumatoid arthritis who presented with grade 2+ inflammation in the superonasal, inferotemporal, and inferonasal quadrants at the baseline visit. She was on stable doses of methotrexate and hydroxychloroquine at the time of enrollment. After one injection, her inflammation improved to grade 1+ in the inferonasal quadrant only and grade 0 in all other quadrants by eight weeks. At week 12, she experienced a flare with grade 1+ in the superotemporal quadrant, 0.5+ superonasally, and 0.5+ inferonasally, meeting criteria for re-injection. She developed an inflammatory reaction at the site of injection four days later (Figure 2).The lesion was incised and drained and sent to pathology which confirmed non-infectious etiology. She was given oral steroids and the inflammation resolved without complication. She chose not to receive further injections and withdrew from the study after the week 32 visit.
Figure 2.
Adverse reaction with subconjunctival sirolimus for anterior scleritis, Participant 4
The left panel shows a sterile inflammatory reaction four days following a subconjuntival sirolimus injection. The right panel shows resolution of the reaction without complication.
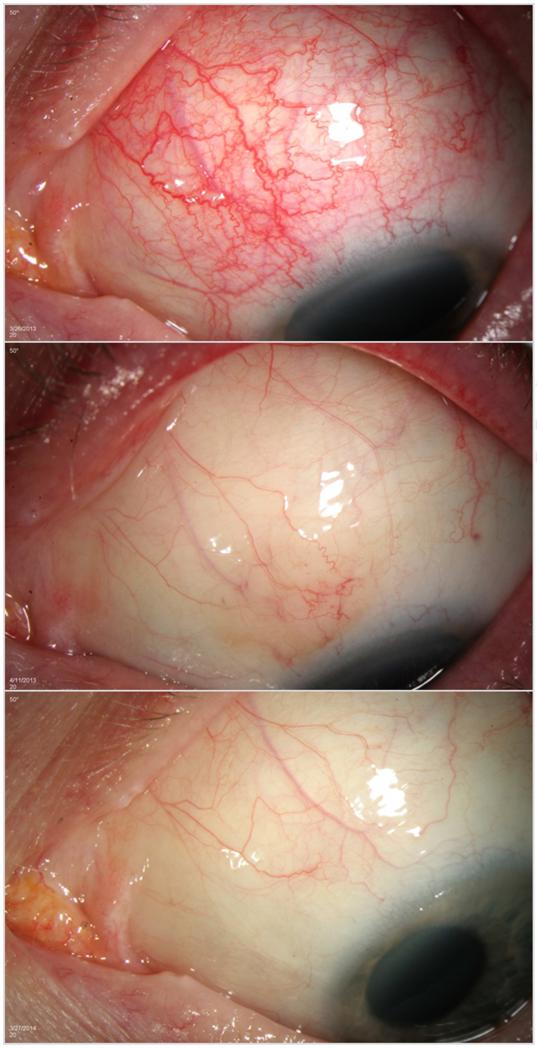
Participant 5 is a 50 year old Caucasian female with a history of systemic lupus erythematosus who presented with grade 3+ inflammation in the superonasal quadrant at the baseline visit. She was on stable doses of adalimumab and low dose prednisone for scleritis at the time of enrollment. After one injection, her inflammation improved to grade 0 in all quadrants after two weeks and remained that way until week 12. At that visit, her scleral inflammation returned to grade 1+ in the superonasal and inferonasal quadrants. She received a repeat subconjunctival sirolimus injection at that visit and subsequently required monthly injections for persistent inflammation for four months until week 32, for total of five injections during the study. After her last injection at week 32, she developed an inflammatory reaction at the site of the injection that was noted by her primary ophthalmologist. She was started on a brief course of oral and topical corticosteroids and the inflammatory reaction resolved without complication. She maintained grade 0 in all quadrants for the remainder of the study. Given her improvement in scleral inflammation and lack of systemic symptoms, adalimumab was successfully discontinued at week 40 (Figure 3).
Figure 3.
Response to treatment with subconjunctival sirolimus for the treatment of Autoimmune Non-Necrotizing Anterior Scleritis, Participant 5
The top panel, middle panel, and bottom panel show Participant 5 at Baseline, Week 2, and Week 52, respectively.
Ophthalmic data collected, including visual acuity, intraocular pressure, and scleral grading is recorded along with patient consent forms at the National Institutes of Health.
Discussion
Scleritis may require systemic therapy including corticosteroids or immunosuppression, all of which can carry significant side effects.8, 9, 17-19 In scleritis, local treatments such as topical medications and periocular injections have been used in the absence of associated systemic disease, but they can be associated with corticosteroid-induced elevated intraocular pressure and cataract. Additionally, local corticosteroid treatments are still considered controversial due to concern for scleral melting, though recent studies suggest long term safety with no reports of scleral melt. In a large multicenter retrospective study, subconjunctival corticosteroid injections were successfully used to treat scleritis in 53 patients with a median follow-up of 2.3 years (range: 6 months to 8.3 years). While subconjunctival injections achieved immediate success in 97% of patients and maintained success beyond one year in 67%, approximately 20% of patients developed ocular hypertension.20 Topical treatments are often not enough and patients have difficulty complying with drop regimens that exceed three times a day. Therefore, a longer acting, locally administered form of therapy that spares patients from systemic side effects would be desirable.
The efficacy of orally administered sirolimus (Rapamune; Wyethe Pharmaceuticals Inc, Radnor, Pennsylvania, USA) was previously reported for refractory uveitis.21,22 It was successful in controlling inflammation and improving macular edema, but intolerable side effects limited the use of high doses. Subconjunctival sirolimus has been used and reported for the same indication in recent trials.23, 24 Results of a recent randomized clinical study assessing the efficacy of intravitreal and subconjunctival injections of sirolimus in patients with non-infectious uveitis suggest a statistically significant reduction in inflammatory indices in both treatment groups at six months, with a good safety profile.24 Subconjunctival sirolimus has not been previously evaluated for the treatment of scleritis.
In this phase I/II trial, subconjunctival sirolimus injections were generally well tolerated systemically and induced a short-term reduction in scleritis severity, with all participants achieving the primary endpoint by eight weeks. We found no correlation between initial disease severity and need for re-injection or total number of injections required due to disease flare. However, the size of our cohort would be insensitive to ascertain such associations.
The most common side effect was a sterile inflammatory reaction at the site of the subconjunctival injection. Previous rabbit studies revealed a similar dose-dependent local toxicity, mainly manifesting as an inflammatory component, and these investigations demonstrated that the adverse effects are largely attributable to the vehicle components (PEG). Whether high doses of sirolimus itself causes some local toxicity and/or exacerbates the effects of the vehicle remains debatable.25 This was reported in previous work with subconjunctival sirolimus for the treatment of anterior uveitis and diabetic macular edema.24, 26 However, no systemic toxicities were observed. Local administration of sirolimus offers the advantage of sparing patients from side effects of systemic therapy while providing a local anti-inflammatory effect. Additionally, it appears to have no ocular hypertensive or cataractogenic side effects.24 Though our study was only one year duration and cataracts can occur in the long term, biologically, sirolimus is not known to have any such effect. Even though most participants required re-injections, the average time to re-injection was approximately two months, ranging between 1-3 months.
Our study is the first study to evaluate this novel non-corticosteroid local injection for the treatment of non-infectious, non-necrotizing anterior scleritis. This local treatment option may potentially avoid cataract and glaucoma induction, which commonly occurs with corticosteroid use. We have observed one year of follow-up with our patients, which is relatively long study duration. Limitations of our study include a small cohort, and a larger study would provide more evidence regarding both safety and efficacy.
In summary, subconjunctival sirolimus may provide a local treatment option which alleviates the issue of patient compliance with frequent eye drops, and potentially spares patients from the effects of systemic immunosuppression.
Acknowledgements
Funding/Support: This research was supported by the National Eye Institute (NEI) Intramural Research Program.
Other acknowledgements: none
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure
Financial Disclosures: None. The study drug was provided in-kind by Santen, Inc.
Contributions to Authors in each of these areas:
Design and conduct of the study (HNS); Collection (NB, MD, WT, DO, HNS), management, analysis and interpretation of the data (NB, RN, HNS), preparation, review or approval of the manuscript (NB, MD, WT, DO, RN, HNS).
References
- 1.Rothova A, Suttorp-van Schulten MS, Treffers WF, Kijlstra A. Causes and frequency of blindness in patients with intraocular inflammatory disease. Br J Ophthalmol. 1996;80(4):332–336. doi: 10.1136/bjo.80.4.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jabs DA, Mudun A, Dunn JP, Marsh MJ. Episcleritis and scleritis: clinical features and treatment results. Am J Ophthalmol. 2000;130(4):469–476. doi: 10.1016/s0002-9394(00)00710-8. [DOI] [PubMed] [Google Scholar]
- 3.Watson PG, Hayreh SS. Scleritis and episcleritis. Br J Ophthalmol. 1976;60(3):163–191. doi: 10.1136/bjo.60.3.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Whitcup S. Scleritis. In: Nussenblatt RB, Whitcup S, editors. Uveitis: Fundamentals and Clinical Practice. 4th Edition Elsevier Inc; 2010. [Google Scholar]
- 5.Fong LP, Sainz de la Maza M, Rice BA, Kaupferman AE, Foster CS. Immunopathology of scleritis. Ophthalmology. 1991;98(4):472–479. doi: 10.1016/s0161-6420(91)32280-2. [DOI] [PubMed] [Google Scholar]
- 6.Bernauer W, Watson PG, Daicker B, Lightman S. Cells perpetuating the inflammatory response in scleritis. Br J Ophthalmol. 1994;78(5):381–385. doi: 10.1136/bjo.78.5.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pasadhika S, Kempen JH, Newcomb CW, et al. Azathioprine for ocular inflammatory diseases. Am J Ophthalmol. 2009;148(4):500–509. doi: 10.1016/j.ajo.2009.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hakin KN, Ham J, Lightman SL. Use of cyclosporin in the management of steroid dependent non-necrotising scleritis. Br J Ophthalmol. 1991;75(6):340–341. doi: 10.1136/bjo.75.6.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sen HN, Suhler EB, Al-Khatib SQ, Djalilian AR, Nussenblatt RB, Buggage RR. Mycophenolate mofetil for the treatment of scleritis. Ophthalmology. 2003;110(9):1750–1755. doi: 10.1016/S0161-6420(03)00570-0. [DOI] [PubMed] [Google Scholar]
- 10.Jachens AW, Chu DS. Retrospective review of methotrexate therapy in the treatment of chronic, noninfectious, nonnectrotizing scleritis. Am J Ophthamol. 2008;145(3):487–492. doi: 10.1016/j.ajo.2007.11.010. [DOI] [PubMed] [Google Scholar]
- 11.Albini TA, Zamir E, Read RW, Smith RE, See RF, Rao NA. Evaluation of subconjunctival triamcinolone for nonnecrotizing anterior scleritis. Ophthalmology. 2005;112(10):1814–1820. doi: 10.1016/j.ophtha.2005.05.008. [DOI] [PubMed] [Google Scholar]
- 12.Sen HN, Ursea R, Nussenblatt RB, Buggage RR. Subconjunctival corticosteroid injection for the treatment of non-necrotising anterior scleritis. Br J Ophthalmol. 2005;89(7):917–929. doi: 10.1136/bjo.2004.052738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Johnson KS, Chu DS. Evaluation of sub-Tenon triamcinolone acetonide injections in the treatment of scleritis. Am J Ophthalmol. 2010;149(1):77–81. doi: 10.1016/j.ajo.2009.07.035. [DOI] [PubMed] [Google Scholar]
- 14.Sehgal SN. Rapamune (RAPA, rapamycin, sirolimus): mechanism of action immunosuppressive effect results from blockade of signal transduction and inhibition of cell cycle progression. Clin Biochem. 1998;31(5):335–40. doi: 10.1016/s0009-9120(98)00045-9. [DOI] [PubMed] [Google Scholar]
- 15.Rapamune [package insert] Wyeth Pharmaceuticals Inc; Philadelphia (PA): Jul, 2008. [Google Scholar]
- 16.Sen HN, Sangave AA, Goldstein DA, et al. A standardized grading system for scleritis. Ophthalmology. 2011;118(4):768–71. doi: 10.1016/j.ophtha.2010.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Foster CS, Forstot SL, Wilson LA. Mortality rate in rheumatoid arthritis patients developing necrotizing scleritis or peripheral ulcerative keratitis.Effects of systemic immunosuppression. Ophthalmology. 1984;91(10):1253–63. doi: 10.1016/s0161-6420(84)34160-4. [DOI] [PubMed] [Google Scholar]
- 18.Shah SS, Lowder CY, Schmitt MA, Wilke WS, Kosmorsky GS, Meisler DM. Low-dose methotrexate therapy for ocular inflammatory disease. Ophthalmology. 1992;99(9):1419–23. doi: 10.1016/s0161-6420(92)31790-7. [DOI] [PubMed] [Google Scholar]
- 19.Jabs DA, Rosenbaum JT, Foster CS, et al. Guidelines for the use of immunosuppressive drugs in patients with ocular inflammatory disorders: recommendations of an expert panel. Am J Ophthalmol. 2000;130(4):492–513. doi: 10.1016/s0002-9394(00)00659-0. [DOI] [PubMed] [Google Scholar]
- 20.Sohn EH, Wang R, Read R, et al. Long-term, multicenter evaluation of subconjunctival injection of triamcinolone for non-necrotizing, noninfectious anterior scleritis. Ophthalmology. 2011;118(10):1932–7. doi: 10.1016/j.ophtha.2011.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shanmuganathan VA, Casely EM, Raj D, et al. The efficacy of sirolimus in the treatment of patients with refractory uveitis. Br J Ophthalmol. 2005;89(6):666–669. doi: 10.1136/bjo.2004.048199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Phillips BN, Wroblewski KJ. A retrospective review of oral low-dose sirolimus (rapamycin) for the treatment of active uveitis. J Ophthalmic Inflamm Infect. 2011;1(1):29–34. doi: 10.1007/s12348-010-0015-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sen HN, Larson TA, Meleth AD, Smith WM, Nussenblatt RB. Subconjunctival sirolimus for the treatment of chronic active anterior uveitis: results of a pilot trial. Am J Ophthalmol. 2012;153(6):1038–1042. doi: 10.1016/j.ajo.2011.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nguyen QD, Ibrahim MA, Watters A, et al. Ocular tolerability and efficacy of intravitreal and subconjunctival injections of sirolimus in patients with non-infectious uveitis: primary 6-month results of the SAVE Study. J Ophthalmic Inflamm Infect. 2013;3(1):32. doi: 10.1186/1869-5760-3-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Watsky MA, Jablonski MM, Edelhauser HF. Comparison of conjunctival and corneal surface areas in rabbit and human. Cur Eye Res. 1998;7(5):483–486. doi: 10.3109/02713688809031801. [DOI] [PubMed] [Google Scholar]
- 26.Dugel PU, Blumenkranz MS, Haller JA, et al. A randomized, dose-escalation study of subconjunctival and intravitreal injections of sirolimus in patients with diabetic macular edema. Ophthalmology. 2012;119(1):124–131. doi: 10.1016/j.ophtha.2011.07.034. [DOI] [PubMed] [Google Scholar]