Fig. 6.
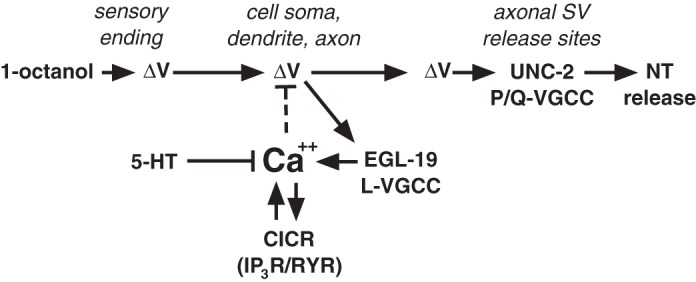
Model of a hypothetical Ca2+-driven negative feedback loop controlling ASH excitability. The activation of the ASH sensory neuron by exposure to 1-octanol involves an as-yet-unidentified amphidial signal transduction cascade, resulting in a membrane depolarization (ΔV), which spreads down the ASH dendrite to the soma and axon, probably passively, given the apparent isopotentiality of C. elegans neurons (Goodman et al. 1998). The amphidial ASH signal transduction cascade activated by other stimuli includes the ODR-3 G protein and polyunsaturated fatty acid signaling to activate the cationic OSM-9/OCR-2 transient receptor potential channels (Bargmann 2006; Colbert et al. 1997; Kahn-Kirby et al. 2004; Roayaie et al. 1998), and these components are also probably involved in activation by 1-octanol. The stimulus-dependent ASH depolarization activates L-type VGCCs (EGL-19) in dendritic, somal, and axonal membranes, and P/Q-type VGCCs (UNC-2) at synaptic vesicle (SV) release sites in the axon, leading to glutamate release. Ca2+ influx through EGL-19 leads to a rise in cytoplasmic Ca2+ that is further amplified by the Ca2+-dependent release of Ca2+ from endogenous stores through both the RYR (UNC-68) and IP3R (ITR-1). This cytoplasmic Ca2+ pool can potentially drive neuronal repolarization by activating Ca2+-dependent inhibitory ion channels (Ca2+-activated K+ channels, anoctamins, or bestrophins; dashed line). Importantly, ASH 5-HT signaling through the SER-5 receptor has the potential to regulate ASH excitability at many levels, including the modulation of ASH Ca2+ dynamics. CICR, calcium-induced calcium release; NT, neurotransmitter.
