Fig. 5.
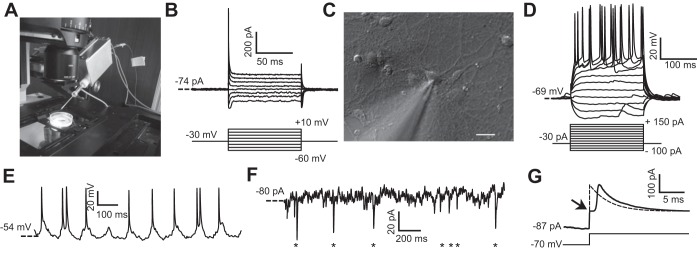
In vitro PatchChip performance. A: PatchChip (inside aluminum box with blue interface cables) with glass pipette electrode connected. The blue cables convey power and digital data between the PatchChip circuitry and a host computer. The dish contains cultured HEK293FT cells. The PatchChip module is attached to a 3-axis micropositioner that allows the electrode tip to precisely contact a cell. B: HEK293FT voltage-clamp experiments using the PatchChip. Ionic currents were recorded in whole cell mode (top) in response to different command voltages (bottom). Current levels and time constants are consistent with typical HEK293FT cell characteristics. C: microscope view showing the tip of the glass pipette contacting a cultured neuron. The pipette was connected to the PatchChip, and voltage- and current-clamp experiments were performed on the cell. Scale bar, 10 μm. D: current-clamp experiment using the PatchChip. Action potentials were evoked when the injected current exceeded 75 pA. E: in vitro current-clamp recordings from cultured neurons using the PatchChip. A clamping current of −30 pA was injected into the cell. Spontaneous action potentials firing at a physiological rate are visible in the measured voltage waveform. F: voltage-clamp experiment using the PatchChip. The cell was held near its resting potential of −70 mV. Miniature postsynaptic currents in the picoamp range from spontaneous activity are visible (asterisks). G: crossover distortion in the voltage-clamp circuit when measured current switched direction. Passive membrane characteristics of a neuron were measured by using sodium channel blockers and stepping the control voltage from −70 to −40 mV. Arrow indicates a “kink” artifact lasting ∼1.4 ms that occurred due to crossover distortion. The ideal passive response of a cell to this voltage step is a first-order exponential (dashed).
