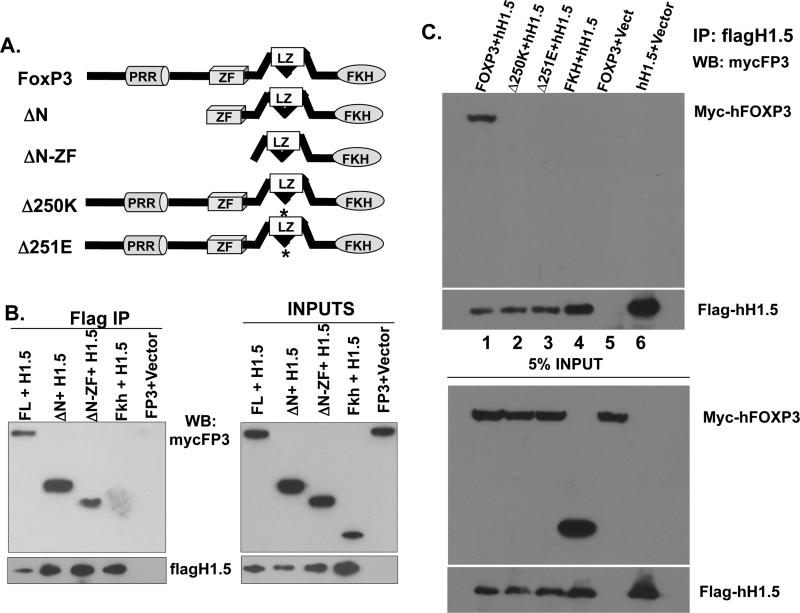
FIGURE 2. FoxP3 specifically interacts with linker histone H1.5 via critical residues in the leucine zipper domain.
(A) Diagrams of the FoxP3 protein domain structure and deletion mutants31. FL=full length, ΔN=N-terminus deletion, ΔN-ZF= deleted N-terminus and zinc finger, Fkh=deleted N-terminus, zinc finger, and leucine zipper. Δ250K/Δ251E=mutant FoxP3 alleles isolated from IPEX patients. (B) Nuclear extracts from 293T cells transfected with flagH1.5 and mycFoxP3 deletion mutants were IPed with anti-flag antibodies and co-IPed mycFoxP3 proteins were detected by immunoblotting. (C) The amino acid residues 250K/251E in leucine zipper domain of FoxP3 are both critical for binding to H1.5, as well as for FoxP3 function. Nuclear extracts from 293T cells transfected with flagH1.5 and human FoxP3 (lane 1), Δ250K (lane 2) or Δ251E (lane 3), the Forkhead domain (lane 4), FoxP3 only (lane 5), and flagH1.5 only (lane 6). Flag-H1.5 was immuno-precipitated with anti-flag antibodies and Western blotted with the indicated antibodies. Inputs represent 5% of cell lysates used for each co-IP experiment.