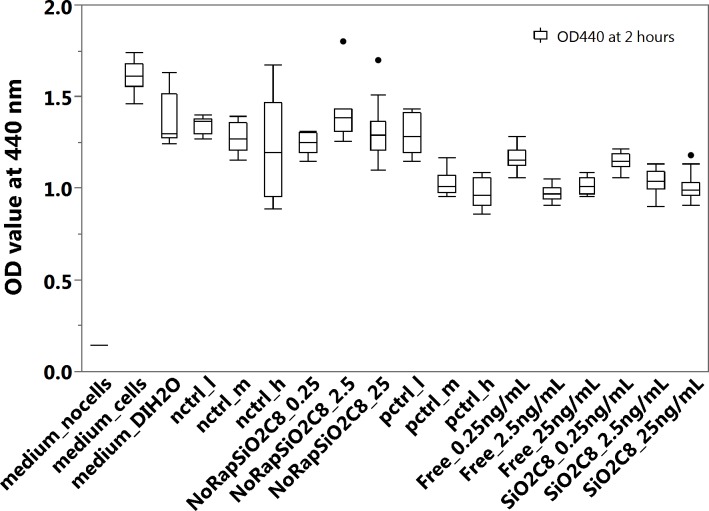
Figure 9.
Viability of EA.hy926 cells determined by WST-1 assay after exposure of cells to the liquid extracts of RAP released from the pSiO2-C8-RAP microparticle formulation or controls. Medium_no cells: 100% culture medium without cells; medium_cells: 100% culture medium and EA.hy926 cells; medium_DIH2O: the cells were treated with a mixture of 75% culture medium and 25% DI H2O; for pctrl_l (positive control, low dose), pctrl_m (positive control, middle dose), and pctrl_h (positive control, high dose), the cells were treated with a mixture of 25% (by volume) of RAP solution and 75% culture medium, at final RAP concentrations of 0.25 ng/mL (low), 2.5 ng/mL (middle), and 25 ng/mL (high). For nctrl_l (negative control, low dose), nctrl_m (negative control, middle dose), and nctrl_h (negative control, high dose), the cells were treated with a mixture of 75% (by volume) culture medium and 25% DI H2O containing the equivalent amount of DMSO contained in the positive controls at concentrations of 0.25 ng/mL (low), 2.5 ng/mL (middle), and 25 ng/mL (high). For Free_0.25ng/mL, Free_2.5ng/mL, and Free_25ng/mL, the cells were treated with a mixture of 25% (by volume) extracts released from free crystalline RAP diluted to final calculated concentrations of 0.25 ng/mL, 2.5 ng/mL and 25 ng/mL, and 75% culture medium. For NoRapSiO2C8_0.25, NoRapSiO2C8_2.5, and NoRapSiO2C8_25, the cells were treated with a mixture of 75% (by volume) culture medium and 25% of extracts released from empty pSiO2-C8 (no drug) formulation following the same dilution protocol as for the positive control samples containing RAP concentrations of 0.25 ng/mL, 2.5 ng/mL, and 25 ng/mL. For SiO2C8_0.25ng/mL, SiO2C8_2.5ng/mL, and SiO2C8_25ng/mL, the cells were treated with a mixture of 75% (by volume) culture medium and 25% of extracts released from the pSiO2-C8 formulation diluted to 0.25 ng/mL, 2.5 ng/mL, and 25 ng/mL.