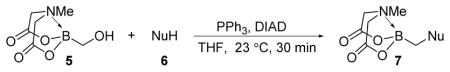
Table 1.
Synthesis of α-functionalized alkyl(MIDA)boronatesa
| |||
|---|---|---|---|
| entry | NuH | product | yield (%) |
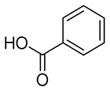
| 1b |
6a
|
7a
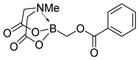
|
99 |
| 2b |
6b
|
7b
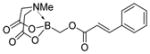
|
94 |
| 3 |
6c
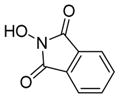
|
7c
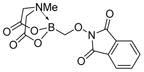
|
97 |
| 4c |
6d
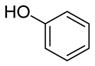
|
7d
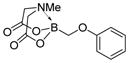
|
91 |
| 5 |
6e
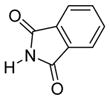
|
7e
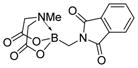
|
99 |
| 6 |
6f
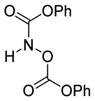
|
7f
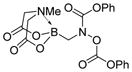
|
78 |
| 7 |
6g
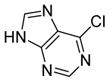
|
7g
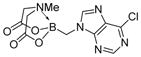
|
98 |
| 8 |
6h
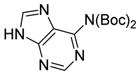
|
7h
|
96 |
| 9 |
6i
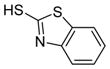
|
7i
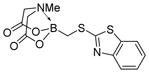
|
85 |
| 10 |
6j
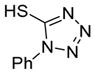
|
7j
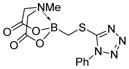
|
92 |
Unless otherwise noted, reactions were conducted with alcohol (1.0 equiv.), 1.0 equiv. of acidic pronucleophile, 1.2 equiv. of triphenylphosphine, and 1.2 equiv. of DIAD in THF at 23 °C for 30 min.
1.5 equiv. of acidic pronucleophile, 1.5 equiv. of triphenylphosphine, and 1.5 equiv. of DIAD were used. Reaction time: 30 min. Temp.: 23 °C.
2.0 equiv. of acidic pronucleophile, 1.5 equiv. of triphenylphosphine, and 1.5 equiv. of DIAD were used. Reaction time: 12 h. Temp.: 23 °C.
